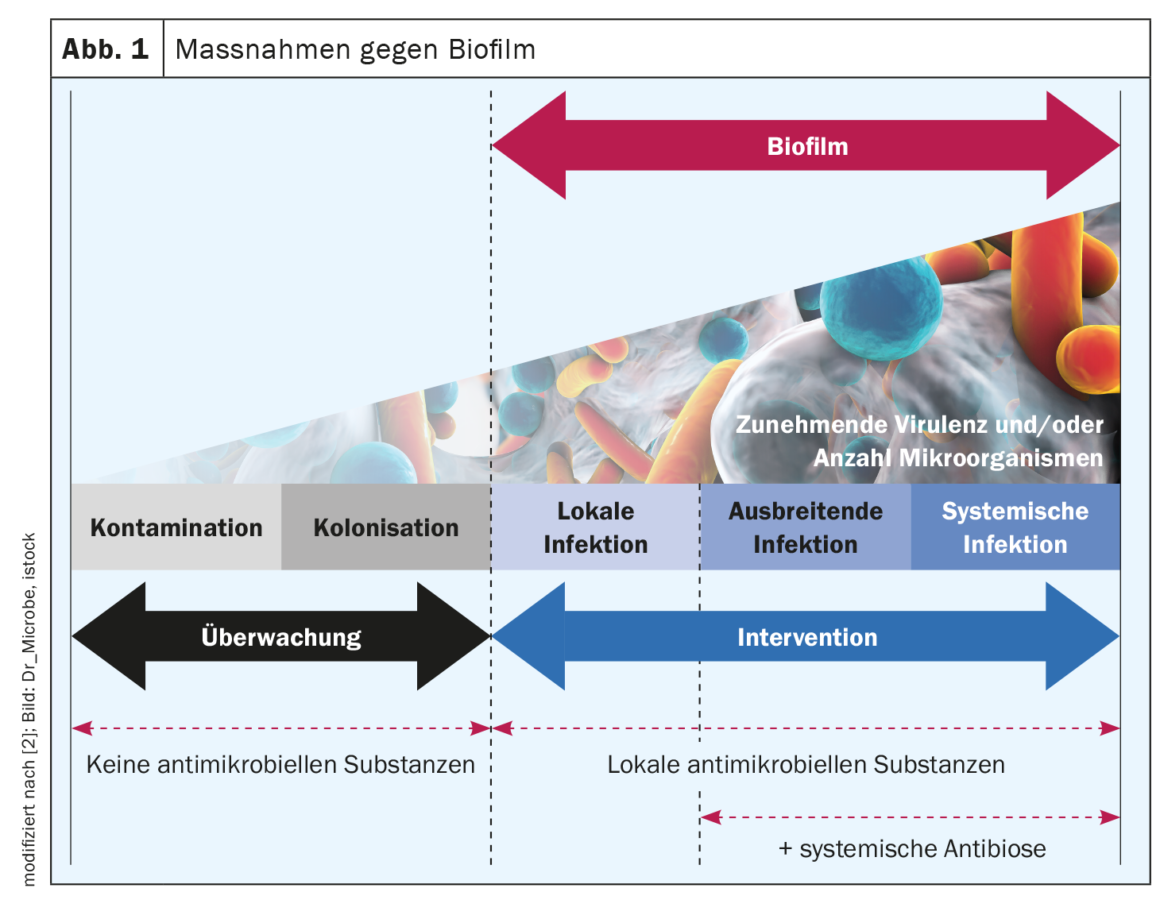
If biofilm colonization persists in chronic wounds despite the best possible treatment of the underlying disease, this causes wound healing to stagnate. Mechanical debridement is extremely important, but is not sufficient as a sole measure and is also impractical in some cases. For antimicrobial wound irrigation solutions and dressings to be effective against biofilm, it is critical that they succeed in penetrating the extracellular polymeric substance. Accordingly, the effectiveness of the various preparations varies.
Wounds that are still open after 30 days are considered chronic regardless of their cause and are susceptible to infection due to several factors [1]. Thus, the warm, moist wound environment is an ideal breeding ground for bacteria and fungi, and delayed wound closure increases the risk of ongoing pathogen exposure with the potential for biofilm formation. On the occasion of this year’s Wound Congress in Nuremberg, Prof. Dr. med. Ewa Klara Stürmer, Medical Director Comprehensive Wound Center and Head of Translational Wound Research, University Medical Center Hamburg-Eppendorf, spoke about clinical practice and new findings in the treatment of wound biofilm [2].

Biofilm is found on over three quarters of all chronic wounds
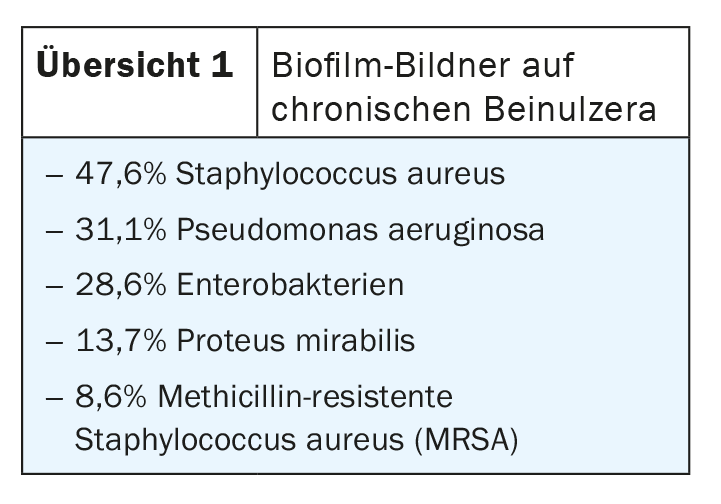
Biofilm occurs when bacterial colonization in the wound results in colonies or bacterial associations in which the bacteria mature and spread (box) [16]. According to a meta-analysis, about 78% of all chronic wounds are colonized with pathogenic microorganisms in the form of biofilms [3]. The wound biofilm locally provides a durable, more or less pronounced immune response or inflammation [4]. If the biofilm is not broken up and eliminated, wound healing stagnates, resulting in the persistence of chronicity of a wound – even with the best possible treatment of the underlying disease [2,4,5]. The leading bacterial species in wound biofilms, using the example of leg ulcers, are (Overview 1): Staphylococcus aureus, its methicillin-resistant variant (MRSA), Pseudomonas aeruginosa, Enterobacteriaceae, and Proteus mirabilis [6].
Extracellular polymeric substance as a critical sticking point
The symbiotic, interspecies society of microorganisms from which biofilms are formed produces an extracellular polymeric substance (EPS) into which they “wall themselves” [2,4]. EPS is mainly composed of polysaccharides as well as a variety of proteins, lipids, glycoproteins, and glycolipids, which makes it firmly adhere to the wound bed [7]. This sugar-protein mixture represents a bacterial “shield” against antiseptics and antibiotics [2]. After 2-4 days, one speaks of “mature biofilm”. A wound biofilm or a strong bacterial colonization of the wound can be made visible to the human eye with UV-near light (e.g. MolecuLight®, MolecuLight Corp., Toronto, Canada) [4].
Efficiency of antimicrobial wound irrigations and dressings
Mechanical debridement is currently the only expert consensus recommended therapy for wound biofilm, but first, debridement alone does not eliminate all biofilm, and second, it is not always a viable option in the home-care setting (e.g., limited hygiene, patients therapeutically anticoagulated with medications) [4,8]. Antimicrobial wound irrigation solutions address and destroy bacterial walls, membranes, and bacterial transport proteins or inhibit their function [4]. If they fail to break through the EPS – which functions as a biochemical barrier against the host immune system and especially against antimicrobial agents – they cannot act effectively. Accordingly, the effectiveness of different wound irrigation solutions against bacterial biofilms varies [4]. Systematic translational testing using the human biofilm model hpBIOM shows that Octenisept® is able to cleave biofilms and kill the bacteria within them within 72 h [4,9,10,17]. PHMB achieves this to a limited extent in the same period, but complete eradication is not achieved even after 72 h. The hypochlorous wound irrigation solutions and chlorhexidine fail to effectively eliminate bacteria in biofilm [4,10].

With regard to the significance of wound dressings, it is somewhat difficult to derive differentiated therapy recommendations from the small evidence base of comparative clinical studies with (antimicrobial) wound dressings, since not only the active substances (silver ions, nanocrystalline silver, PHMB, iodine derivatives, etc.) and their concentrations vary, but also the respective basis of the wound dressings (PU foam, alginates, fibers, etc.). [4,11]. But in multiple in vitro tests using human biofilm model showed that a combination product of iodine and starch (Cadexomer iodine) was able to completely eradicate bacteria in biofilm within six days [2,4]. Other active ingredient wound dressings containing PHMB or silver resulted in only a reduction in bacterial load [12]. The corresponding analyses were performed after a period of six days without dressing changes and it can be assumed that a higher effectiveness is achievable for all of the tested wound dressings if they are changed daily or every two days [4].
The mechanism of action of the combination product of iodine and starch (Iodosorb™)is based on cadexomer microspheres, which destroy the biofilm structure or bacterial extracellular polymeric substance by dehydration as a prerequisite for iodine to readily eliminate the bacteria thus exposed in the biofilm [12,18].
Congress: Wound Congress Nuremberg
Literature:
- Hunt S, Elg F: The clinical effectiveness of haemoglobin spray as adjunctive therapy in the treatment of chronic wounds. J Wound Care 2017; 26(9): 558-568.
- “Biofilm – The Challenge of the Decade in Wound Care – Recognizing, Understanding and Sustainably Eliminating Wound Biofilm,” Prof. Dr. med. Stürmer, Industry Symposium Smith & Nephew GmbH, Wound Congress Nuremberg, Dec. 1, 2022.
- Malone M, et al: The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 2017; 26(1): 20-25.
- Stürmer EK, Rembe J-D: Recognizing and understanding wound biofilm: Therapeutic possibilities and their limitations. CME continuing education, DERMATOLOGIE PRAXIS 2/2022, 6-11.
- James GA, et al: Biofilms in chronic wounds. Wound Repair Regen 2008; 16(1): 3-44.
- Jockenhofer F, et al: Bacteriological pathogen spectrum of chronic leg ulcers: Results of a multicenter trial in dermatologic wound care centers differentiated by regions. J Dtsch Dermatol Ges 2013; 11(11): 1057-1063.
- Flemming HC, Wingender J: The biofilm matrix. Nat Rev Microbiol 2010; 8(9): 623-633.
- Schwartz JA, et al: Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care. 2014 Sep; 23(9): S4, S6, S8 passim. doi: 10.12968/jowc.2014.23.Sup9.S4.
- Besser M, et al: Efficacy of antiseptics in a novel 3-dimensional human plasma biofilm model (hpBIOM). Sci Rep 2020; 10(1): 4792.
- Rembe JD, et al: Antimicrobial Hypochlorous Wound Irrigation Solutions Demonstrate Lower Anti-biofilm Efficacy Against Bacterial Biofilm in a Complex in-vitro Human Plasma Biofilm Model (hpBIOM) Than Common Wound Antimicrobials. Front Microbiol 2020; 11: 564513.
- Schwarzer S, et al: The efficacy of topical agents used in wounds for managing chronic biofilm infections: A systematic review. J Infect 2020; 80(3): 261-270.
- Stuermer EK, et al: In vitro activity of antimicrobial wound dressings on P. aeruginosa wound biofilm. Front Microbiol 2021; 12: 664030.
- Stuermer EK, et al: Bacterial infiltration in biofilm-colonized wounds: Analyses in the hpBIOM ex vivo wound model and possible impact on swabbing and debridement. Int Wound J 2022 (under revision).
- 14. “Principles of Wound Care, Wound Cleansing/Debridement Techniques,” www.bk-trier.de/media-bkt/docs/Bildung/Handouts-Wundmanagement/Basisseminar-Handouts/03_Debridement.pdf,(last accessed Jan. 20, 2023).
- Rembe JD, Stürmer EK: Modern wound antisepsis – indications and limitations, between knowledge, desire and uncertainty. Vascular Surgery 2020; 25, 272-276.
- Percival SL, McCarty SM, Lipsky B: Biofilms and Wounds: An Overview of the Evidence. In: Advances in wound care 2015; 4(7): 373-381.
- Swiss Drug Compendium,
https://compendium.ch,(last accessed 20.01.2023) - Iodosorb™ Dressing, www.smith-nephew.com/switzerland/fachgebiete/wundmanagement/iodosorb-dressing,(last accessed Jan. 20, 2023).
DERMATOLOGIE PRAXIS 2023; 33(1): 32-33 (published 2/16/2013, ahead of print).











