Compared with patients without AF, ischemic insults are significantly more frequent in patients with AF and are associated with more severe impairment and higher mortality. Adequate oral anticoagulation can effectively prevent ischemic insults in patients with AF. The prevalence of subclinical AF is underestimated, but all of the above points apply to subclinical AF as well. The possibility of subclinical atrial fibrillation should always be considered. Clinical, electrical and echocardiographic parameters as well as biomarkers may be indicative of its presence.
Atrial fibrillation is the most common arrhythmia, with a prevalence of 1.5-2.0% in the general population. While the prevalence in 65-year-olds is still around 1%, it rises steeply in the further decades of life and already exceeds 10% in 80-year-olds [1]. Due to the aging of the population, a significant increase in the prevalence of atrial fibrillation is expected in the future.
Atrial fibrillation, morbidity and mortality
Epidemiologic studies have associated the presence of atrial fibrillation with increased mortality [2]. The influence of atrial fibrillation on the incidence of insults was investigated in the Framingham study before the introduction of oral anticoagulation in atrial fibrillation patients: Compared with individuals without atrial fibrillation, individuals with atrial fibrillation had a fivefold increased risk of insult [3]. In addition, compared with patients without AF, insults in patients with AF are more severe, result in higher disability at hospital discharge, and have increased mortality at 30 days as well as at one year [4]. Sufficient oral anticoagulation can effectively prevent ischemic insults in patients with AF. If an ischemic insult occurs in patients with atrial fibrillation despite sufficient anticoagulation, the outcome is not worse than in patients without atrial fibrillation.
The prevalence of AF in patients with ischemic insult is 5-9% in those younger than 60 years and exceeds 40% in those older than 80 years [4]. However, these figures only include previously known atrial fibrillation or atrial fibrillation observed during hospitalization.
Subclinical atrial fibrillation
Atrial fibrillation is usually symptomatic, especially in young and active individuals. However, it is well established that even patients with highly symptomatic AF always have asymptomatic episodes. Asymptomatic episodes are common, especially in elderly patients. Whereas the diagnosis of persistent AF poses few problems, paroxysmal AF is often diagnosed late, possibly even in the setting of a first thromboembolic event, especially in less active patients.
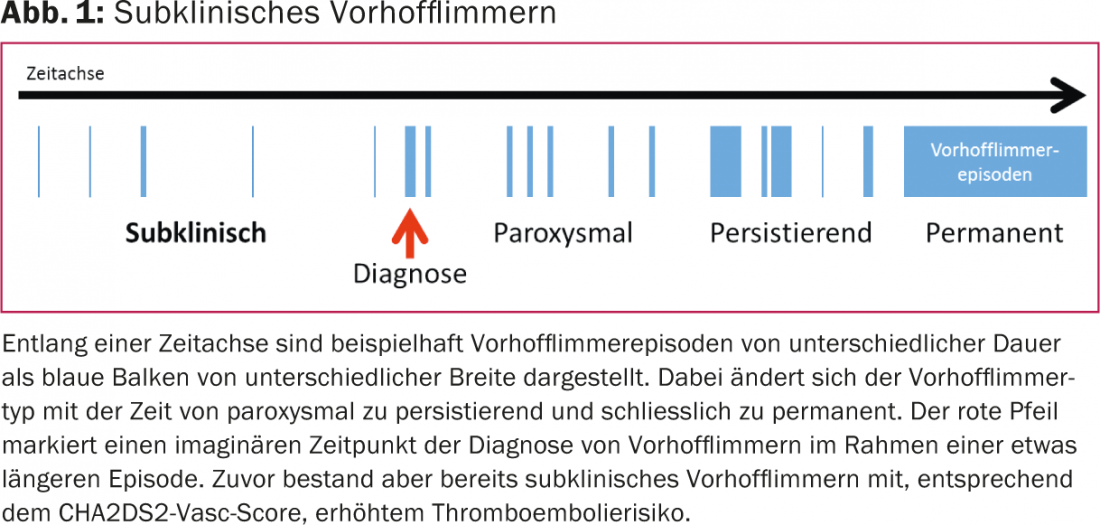
Subclinical atrial fibrillation refers to previously unnoticed and usually asymptomatic atrial fibrillation (Fig. 1).
Recently, two randomized trials appeared in patients with cryptogenic ischemic insult. In both studies, subclinical AF was systematically searched for in one arm each. In one study, an implanted event recorder was able to detect subclinical AF after one year in 12% of patients with a mean age of 61 years [5]. In the other study, subclinical atrial fibrillation was found by 30-day ECG in 15% of patients with a mean age of 73 years [6]. If subclinical atrial fibrillation is also taken into account, the prevalence of atrial fibrillation in patients with ischemic insult should thus already reach 50% at the age of 75 years.
That subclinical atrial fibrillation is indeed associated with an increased risk of thromboembolism has been shown, among others, by the ASSERT study [7]. Patients with pacemakers or defibrillators who also had an atrial electrode implanted were included in this study. Patients who experienced atrial high-frequency episodes during the first 3 months after inclusion were compared with the rest of the study population during 2.5 years for the occurrence of AF or a thromboembolic event. Atrial high-frequency episodes were found in 10% of patients. These had a significantly higher likelihood of also developing clinically manifest AF, and in particular, the rate of thromboembolic events was significantly higher in this group.
Screening for atrial fibrillation
Current guidelines recommend screening for atrial fibrillation by palpating the pulse at every medical visit [1]. If an irregular pulse is detected, a 12-lead ECG should be recorded immediately to confirm the diagnosis of atrial fibrillation. This simple screening method can newly diagnose atrial fibrillation in 1.4% of patients over 65 years of age.
A targeted search for subclinical atrial fibrillation involves ECG recording over an extended period of time. Here, the sensitivity is directly proportional to the duration of ECG recording as well as to the frequency of repetitions [8]. Other important factors affecting sensitivity are arrhythmia load and density, i.e., the total duration and distribution of atrial fibrillation episodes. In addition to continuous ECG recording, event-activated devices are also used. The latter monitor the heart rhythm and record only when predefined events occur (e.g., a heart rate >150/min.) record a short rhythm strip. Furthermore, devices can be used with which the patient can independently record a short rhythm strip several times a day and in the case of corresponding symptoms. This is then sent to the attending physician via mobile radio. In addition to these established medical devices, there is an increasing number of options for rhythm or ECG recording that can be purchased and operated by laypersons, mostly based on smartphone technology. Table 1 provides an overview of the various options for screening for atrial fibrillation.

In a noteworthy study, Engdahl and colleagues invited all 75-76-year-old residents of a small Swedish town to undergo stepwise screening for atrial fibrillation [9]. Atrial fibrillation was already known in 9.6% of the residents. By recording a 12-lead ECG, atrial fibrillation was newly detected in 1.2% of cases. In case of an increased risk of thromboembolism, all remaining participants were searched for atrial fibrillation by means of twice-daily recording of a rhythm strip over a period of two weeks. This allowed additional detection of atrial fibrillation in 7.4% of cases.
This study impressively demonstrates the high prevalence of subclinical AF in patients at increased risk of thromboembolism. Thus, a population with a clear benefit from early oral anticoagulation. However, an obstacle to systematic screening is that it is resource- and time-intensive and inconvenient for patients.
Predictors of (subclinical?) atrial fibrillation.
Various clinical, electrical and echocardiographic parameters as well as biomarkers are correlated with the incidence of AF, resp. differences exist between patients with and without atrial fibrillation.
For example, from data from the Framingham cohort, a risk score was developed that calculates the risk of developing atrial fibrillation over the next decade [10]. Age, sex, body mass index, systolic blood pressure, antihypertensive therapy, PR interval, and the age at which a heart murmur or heart failure first occurred are considered. The number of supraventricular extrasystoles as well as the duration of the longest atrial tachycardia in a 48-hour ECG also correlate very well with the incidence of AF [11]. In addition, patients with atrial fibrillation have a broader P-wave in comparison, which can be measured even more precisely by signal averaging. Echocardiographic evidence shows that patients with atrial fibrillation have larger atrial volumes and there are also differences in diastolic parameters. Furthermore, CRP, BNP, and troponin have been correlated with increased incidence of AF in various studies.
None of these parameters is able to reliably identify patients with atrial fibrillation. Nevertheless, these parameters, especially in combination, could be indicative not only of an increased likelihood of a future AF diagnosis but also of the presence of subclinical AF. Therefore, an alternative strategy to mass screening, as practiced by Engdahl and colleagues [9], would be targeted screening of subpopulations with an increased likelihood of subclinical AF based on the above-mentioned and other parameters.
Until such screening programs find their way into clinical practice, we should remain vigilant and always consider the possibility of subclinical atrial fibrillation in our patients. In addition to regular pulse palpation, we should not hesitate to specifically look for subclinical atrial fibrillation by means of repeated long-term ECGs if there is evidence of it.
Acknowledgements: The Swiss Heart Foundation and the Foundation for Pacemakers and Electrophysiology support a study at Inselspital with the aim of improved screening for subclinical atrial fibrillation.
Literature:
- Camm AJ, et al: Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (esc). Eur Heart J 2010; 31 (19): 2369-2429.
- Stewart S, et al: A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the renfrew/paisley study. Am J Med 2002; 113 (5): 359-364.
- Wolf PA, et al: Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke 1991; 22 (8): 983-988.
- McGrath ER, et al: Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology 2013; 81 (9): 825-832.
- Sanna T, et al: Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370 (26): 2478-2486.
- Gladstone DJ, et al: Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370 (26): 2467-2477.
- Healey JS, et al: Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012; 366 (2): 120-129.
- Charitos EI, et al: A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: Insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 2012; 126 (7): 806-814.
- Engdahl J, et al: Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation 2013; 127 (8): 930-937.
- Schnabel RB, et al: Development of a risk score for atrial fibrillation (framingham heart study): A community-based cohort study. Lancet 2009; 373 (9665): 739-745.
- Binici Z, et al: Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation 2010; 121 (17): 1904-1911.
CARDIOVASC 2014; 13(6): 24-29