Lower respiratory tract infections (LRTIs) caused by respiratory syncytial virus (RSV) contribute significantly to morbidity and mortality in infants 0 to 1 years of age. Although there is a well-documented association between RSV-LRTI and subsequent wheezing illness, it is still unclear whether the association is causal.
Scientists led by Steven M. Brunwasser, M.D., of Vanderbilt University Medical Center, Nashville, Tennessee, set out to analyze and evaluate the strength of evidence for a causal effect of RSV-LRTI on subsequent chronic wheezing illnesses, such as bronchial asthma . To this end, they conducted a systematic review and meta-analysis of observational studies examining the association between RSV-LRTI and subsequent wheezing illness (exposure studies), on the one hand, and of studies evaluating the association between RSV immunoprophylaxis and subsequent wheezing illness (immunoprophylaxis studies), on the other. All variants of wheezing disease were combined into a single outcome that generally refers to asthma or any other respiratory disease with wheezing symptoms.
Although meta-analyses cannot resolve the issue of causality, they can help assess the strength of evidence for causality. If successful, it would be expected that effective RSV-LRTI prevention could likely reduce the burden of chronic disease.
Three models developed
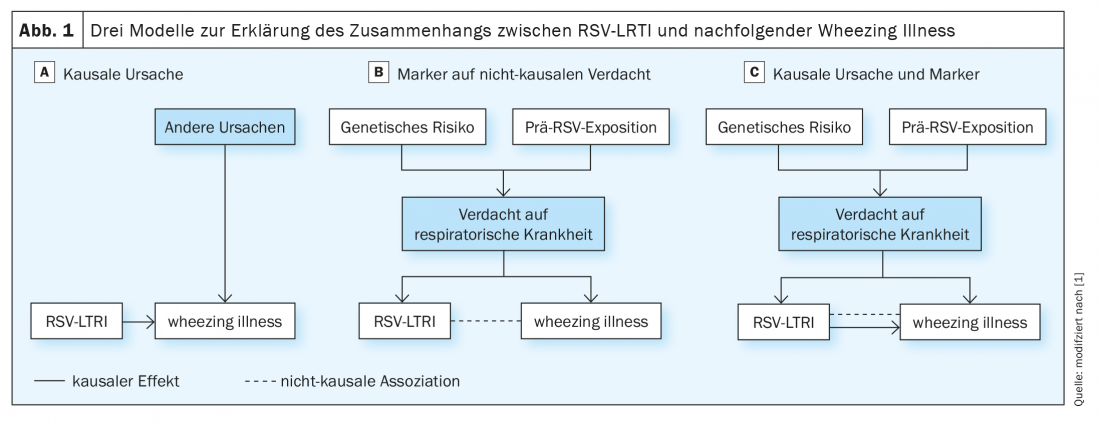
The researchers presented three possible models for the established association between RSV-LRTI and wheezing illness. (Fig.1). In the first model, RSV-LRTI is one of several causative factors for subsequent wheezing illness (Fig. 1A). The second is a noncausal model in which preexisting susceptibility to respiratory disease favors both RSV-LRTI and subsequent wheezing illness (Fig. 1B). In this model, susceptibility to preexisting respiratory disease is due to heritable factors and early environmental influences. Previous research has shown that infants who develop RSV-LRTI have poorer preexisting lung function (a highly heritable trait), which could make them susceptible to both severe illness in response to RSV infection and wheezing illnesses. In the third model, the association is partly due to a causal effect and partly to the confounding influence of preexisting susceptibility to respiratory disease (Fig. 1C).
Among RSV-LRTI exposure studies, OR showed that children exposed to RSV-LRTI were 3.39-fold more likely to have subsequent wheezing illness (95% CI 2.72-4.24). The OR remained positive when the analysis was limited to effect estimates of RSV-LRTIs on asthma outcomes measured at age 6 years and older (41 estimates from 14 studies, OR 2.64; 95% CI 1.75-3.98).
In the investigators’ model, effect estimates for the association between RSV-LRTI and wheezing illness differed depending on whether estimates were adjusted for genetic influences (b 0.53; 95% CI 0.04-1.02). As seen in Figure 2 , the adjusted mean OR (aOR) was significantly smaller when estimates accounted for genetic influences (n=77, aOR 2.45; 95% CI 1.23-4.88) compared with those that did not (n=52, aOR 4.17; 95% CI 2.36-7.37).
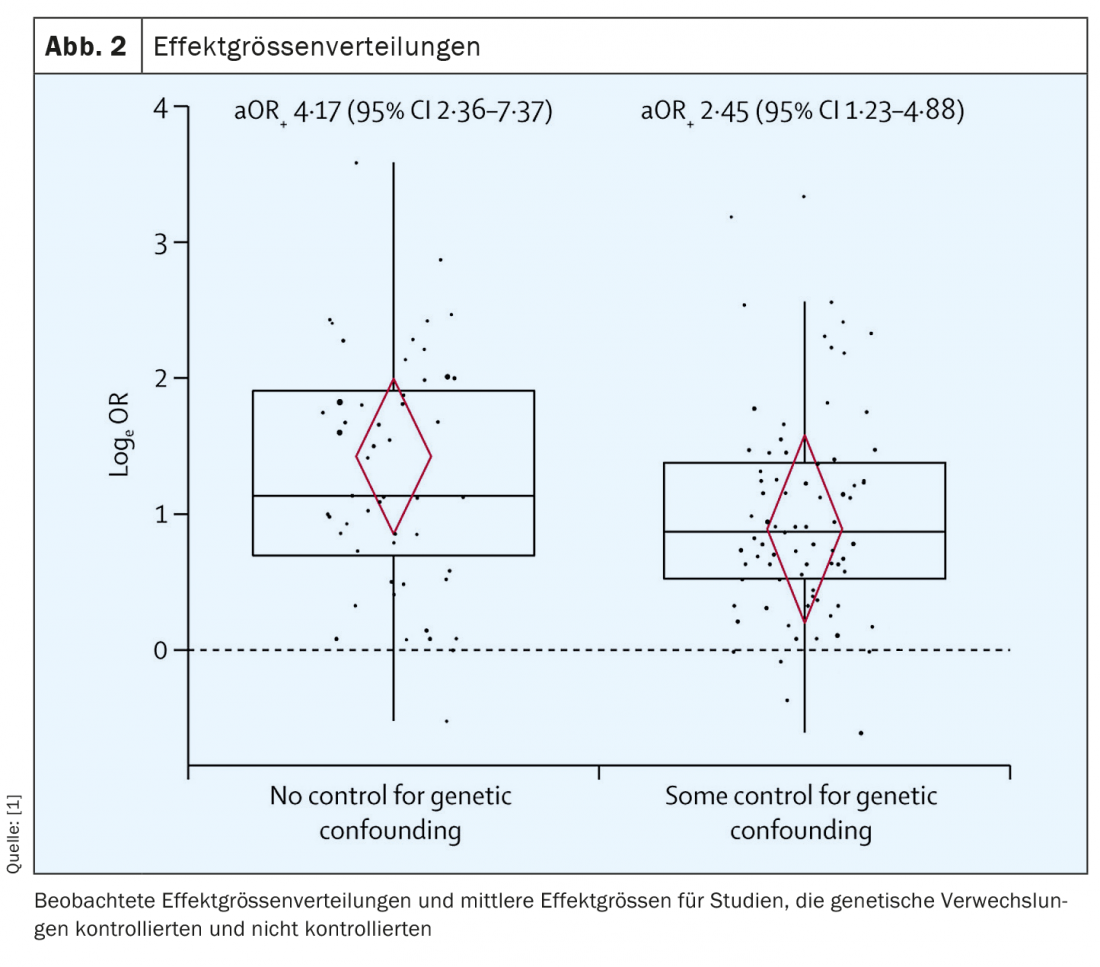
This difference reached statistical significance and shows that genetic influences must definitely be considered in the analysis of potential causality. Unsurprisingly, these appear to play a role in the development of wheezing illness with or without additional RSV infection. It will probably never be possible to completely separate the various causes for the development of bronchial asthma, for example, and their respective significance due to the confounding bias, which the authors of the study also describe.
Sobering result
Although a causal effect of RSV-LRTI on subsequent wheezing illness cannot be excluded, neither model supported the assumption of causality. First, RSV-LRTI exposure studies that controlled for genetic influences yielded smaller effect estimates. The would be consistent with what would be expected if RSV-LRTI were at least in part a marker of genetic susceptibility, rather than a purely causal association. As a consequence, the models likely underestimated the influence of adaptation on genetics, Brunwasser and his colleagues concede. Second, existing immunoprophylaxis studies did not provide convincing evidence that RSV immunoprophylaxis protects against subsequent wheezing illness. Even if children without RSV immunoprophylaxis tended to have a higher risk of developing wheezing illnesses in the included studies, this effect was not statistically significant (OR 1.21; 95% CI 0.73-1.99). Of course, this could also be due to the small number of only eight studies included in the researchers’ analysis. Additional, preferably large, RCTs would improve precision.
In summary, this study, in combination with previous analyses, suggests that existing data do not well support evidence of a causal effect of RSV-LRTI on subsequent wheezing illness. Furthermore, the current evidence cannot strengthen the assumption that effective RSV-LRTI prevention strategies would reduce subsequent wheezing illnesses. Future observational studies evaluating the association between RSV-LRTI and wheezing illness are likely to be helpful in resolving the causality question only if they accurately account for genetic influences, according to Brunwasser et al. With respect to RSV immunoprophylaxis studies, RCTs would likely provide less biased estimates than observational studies.
Not all evidence relevant to assessing whether RSV-LRTI causes wheezing illness could be included because some important studies did not meet the investigators’ inclusion criteria. In addition, all RSV immunoprophylaxis studies were conducted in high-risk populations, which may limit generalizability. Finally, almost all of the data included were from high-income countries, which may make the results unrepresentative of low- and middle-income countries.
Source:
- Brunwasser SM, Snyder BM, Driscoll AJ, et al: Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: a systematic review and meta-analysis. Lancet Respiratory Medicine 2020; 8(8): 795-806; doi: 10.1016/S2213-2600(20)30109-0.
InFo PNEUMOLOGY & ALLERGOLOGY 2020; 2(4): 24-25.