In Switzerland, the approval of the highly effective biologic Dupilumab in the indication area of atopic dermatitis has so far been limited to adult and adolescent patients aged 12 years and older. The monoclonal antibody provides rapid and lasting symptom relief and has been shown to improve the quality of life of atopic dermatitis sufferers. In the EU, an indication extension for the age group 6-11 years was granted a few months ago based on corresponding phase III studies. That dupilumab is also effective and safe in preschool-aged children is demonstrated by recently published data from the LIBERTY AD-PRESCHOOL study.
Patients with atopic dermatitis suffer from a significantly reduced quality of life, especially when the disease is severe. Excruciating itching is very unpleasant, can lead to sleep disturbances and, especially in children and adolescents, can contribute to concentration and learning difficulties. Furthermore, patients feel very stigmatized by visible skin changes. The main goal of the therapy is to relieve the itching and treat the inflammatory skin changes. Guideline-oriented therapy is carried out in a step-adapted manner. System therapy is recommended for persistent, severe eczema that responds inadequately to topical therapeutics.
For 12- to 17-year-olds, weight-adapted dosing regimen.
The monoclonal antibody dupilumab blocks signal transduction of interleukins 4 and 13, whose expression correlates with disease activity in people with atopic dermatitis. Dupilumab (Dupixent®) is available as a pre-filled syringe in the prescribed dosage and can be self-administered subcutaneously by patients after medical instruction [1]. For adults with moderate to severe atopic dermatitis, Dupixent® has been approved in Switzerland since 2019. In November 2020, Swissmedic granted an indication extension for adolescents aged 12 years and older. Dupixent® is indicated when therapy with prescription topical medications does not provide adequate disease control or is not recommended [1]. A condition for reimbursement by the health insurance is the limitatio that a treatment with at least one conventional immunosuppressant did not achieve sufficient effect. In adults, dupilumab is administered at a starting dose of 600 mg (2× 300 mg) followed by 300 mg at two-week intervals. In patients aged 12 to 17 years, the administration interval is also two weeks, and the dosing regimen is weight-adapted: in adolescents with a body weight <60 kg, the initial dose is 400 mg (2× 200 mg), then 200 mg at 2-week intervals. In adolescents with a body weight ≥60 kg, an initial dose of 600 mg (2× 300 mg) is recommended, followed by 300 mg at two-week intervals [1].
Already approved in the EU for 6- to 11-year-olds and
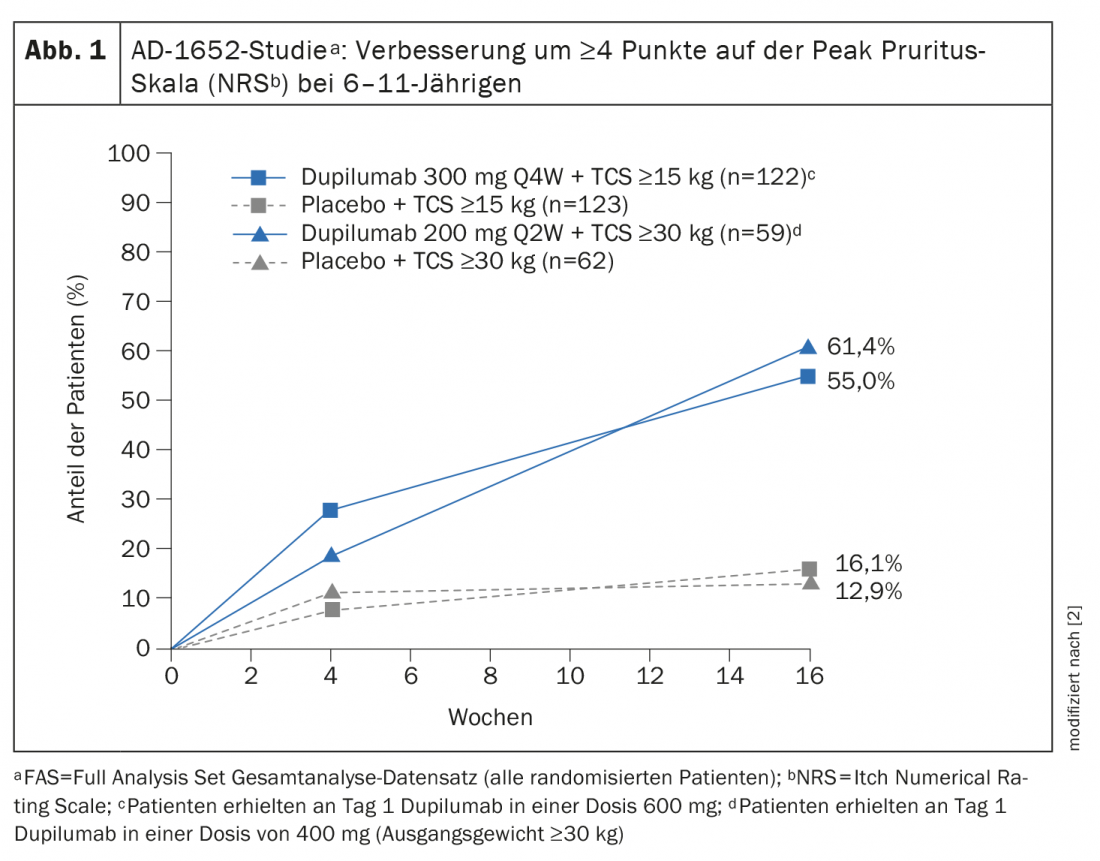
The European Commission granted approval to dupilumab for children 6 years and older in November 2020 [2,3]. The indication expansion is based on data from the randomized, double-blind, controlled study AD-1652, which evaluated the efficacy and safety of dupilumab in combination with TCS in 367 patients aged 6 to 11 years. The results of key efficacy endpoints can be seen in Table 1 [2]. The coprimary endpoint was the proportion of patients with IGA* 0 or 1 (appearance-free or near-appearance-free) with an improvement of ≥2 points and the proportion of patients with EASI-75 (EASI improvement of at least 75%) between baseline and week 16. Results stratified by baseline weight showed for study participants with a KG of 15-29.9 kg treated with dupilumab 300 mg Q4Wd#+ TCS, 32.8% had appearance-free or near-appearance-free skin (IGA 0 or 1), whereas this proportion was 11.4% in study participants treated with placebo plus TCS. In patients ≥30 kg who received 200 mg dupilumab every two weeks, this was as high as 39.0% versus 9.7%. A 75 percent improvement in their skin appearance according to the Eczema Area and Severity Index (EASI-75) was achieved by 69.7 percent and 74.6 percent of dupilumab-treated patients, respectively, compared with 26.8 percent and 25.8 percent in the corresponding placebo groups. Dupilumab+TCS also proved clearly superior to placebo+TCS in terms of reduction in SCORAD and improvement in NRS. More than half of the patients in the dupilumab groups achieved an improvement of ≥4 points in the NRS, whereas in the placebo groups this proportion was 12.9% resp. 16.1% (Fig. 1).
* IGA = Investigator’s Global Assessment
#
Q4Wd = dosing interval of 4 weeks
Patients aged 6 months to 5 years: promising new data
Atopic dermatitis most commonly begins between 3 and 6 months of age, with 60% of patients developing the disease in the 1st year of life. By 5 years of age, disease onset occurs in 90% of patients [4]. The Phase III LIBERTY AD PRESCHOOL study confirmed the well-established efficacy and safety profile of Dupixent® in other age groups in children aged 6 months to 5 years with moderate to severe atopic dermatitis. All primary and secondary endpoints were met. The data show that the addition of dupilumab to standard treatment with topical corticosteroids (TCS) significantly reduced overall disease severity and improved skin clearance, itch, and health-related quality of life after 16 weeks compared with TCS alone. During the 16-week treatment period, the overall rate of adverse events (AEs) was 64% for dupilumab and 74% for placebo. The most common UAEs included nasopharyngitis (8% dupilumab, 9% placebo), upper respiratory tract infections (6% dupilumab, 8% placebo) and conjunctivitis (5% dupilumab, 0% placebo), herpes viral infections (6% dupilumab, 5% placebo), and injection site reactions (2% dupilumab, 3% placebo). In summary, the safety results of this study were consistent with the known safety profile of dupilumab in atopic dermatitis.
Literature:
- Brief technical information: Dupixent®, www.swissmedicinfo.ch (last accessed Nov. 19, 2021)
- European Medicines Agency (EMA), www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_de.pdf (last accessed Nov. 19, 2021).
- “Dupixent® (dupilumab) approved by European Commission as first and only biologic medicine for children aged 6 to 11 years with severe atopic dermatitis”, Sanofi, 11/30/2021.
- Barbarot S, et al: Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018; 73(6): 1284-1293.
DERMATOLOGIE PRAXIS 2021; 31(6): 41-42