| In the “BioDay Registry” study, treatment with dupilumab in pediatric patients with atopic dermatitis in a “real world” setting resulted in significant improvement in disease severity and associated symptoms. Tolerability was found to be good, and the safety profile is consistent with results from clinical trials. |
Dupilumab has been shown to be an effective and safe treatment for atopic dermatitis (AD) to date [1]. By binding to the IL-4Rα subunit of the IL-4 and IL-13 receptor complexes, the monoclonal antibody specifically inhibits the proinflammatory effects of these two key cytokines [1]. In Switzerland, dupilumab (Dupixent®) is approved for patients with AD aged 6 years and older [1]. To investigate the treatment effects of dupilumab in pediatric AD patients in a “real world” setting, the “BioDay Registry” study was conducted [2]. In this prospective cohort study, dupilumab-treated children (≥6 to <12 years) and adolescents (≥12 to <18 years) with moderate to severe AD were studied. Patients with a body weight <60 kg received dupilumab (s.c.) every two weeks (q2w) after an initial loading dose of 400 mg. In patients with a body weight ≥60 kg, 600 mg was administered as a loading dose, followed by 300 mg q2w. Children weighing 15-60 kg received 300 mg at baseline and after 2 weeks, and then 300 mg dupilumab every four weeks. Concomitant treatment with topical corticosteroids was allowed.
At the survey time points in week 0 (baseline) and in week 4, 16 and 28 the severity of the disease was assessed by validated scores: “Eczema Area and Severity Index” (EASI), “Investigator Global Assessment” (IGA), “Numeric Rating Scale” (NRS) for itching and pain, “Patient-Oriented Eczema Measure” (POEM) [2]. In addition, serum biomarkers associated with severity were measured.
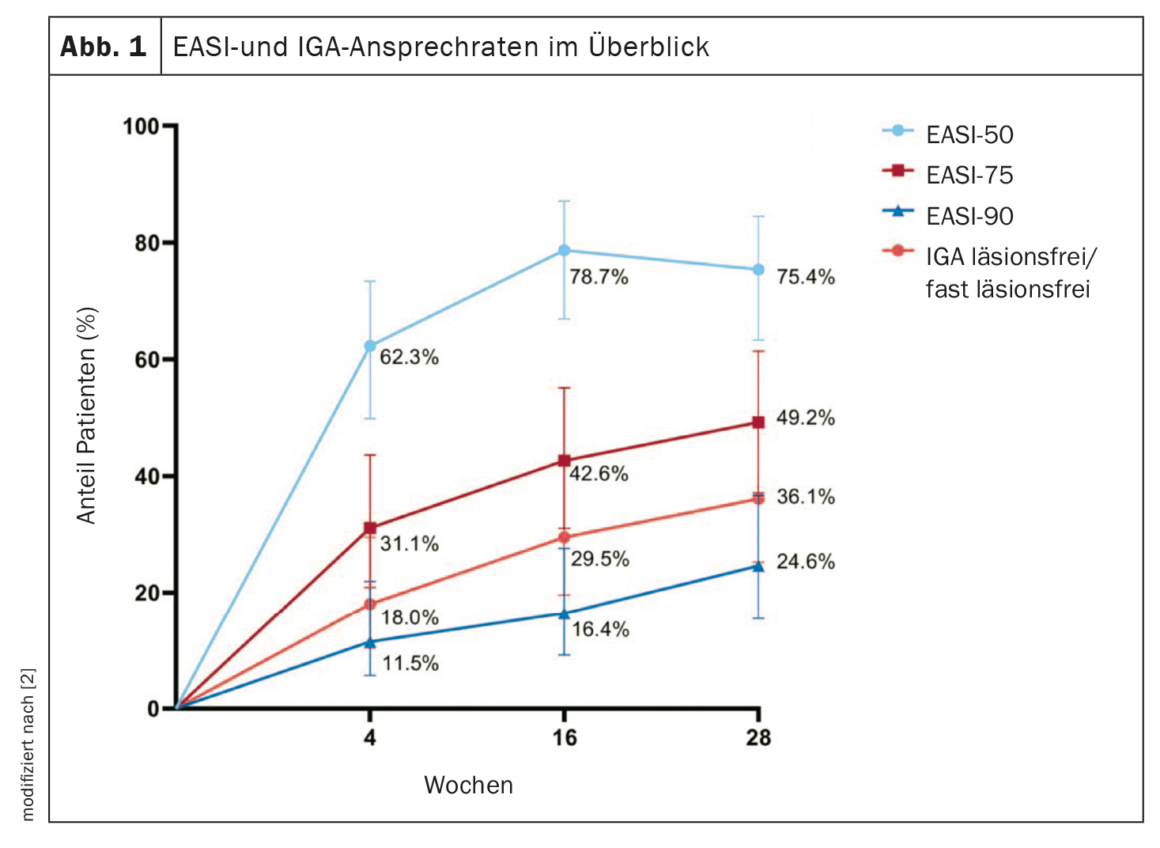
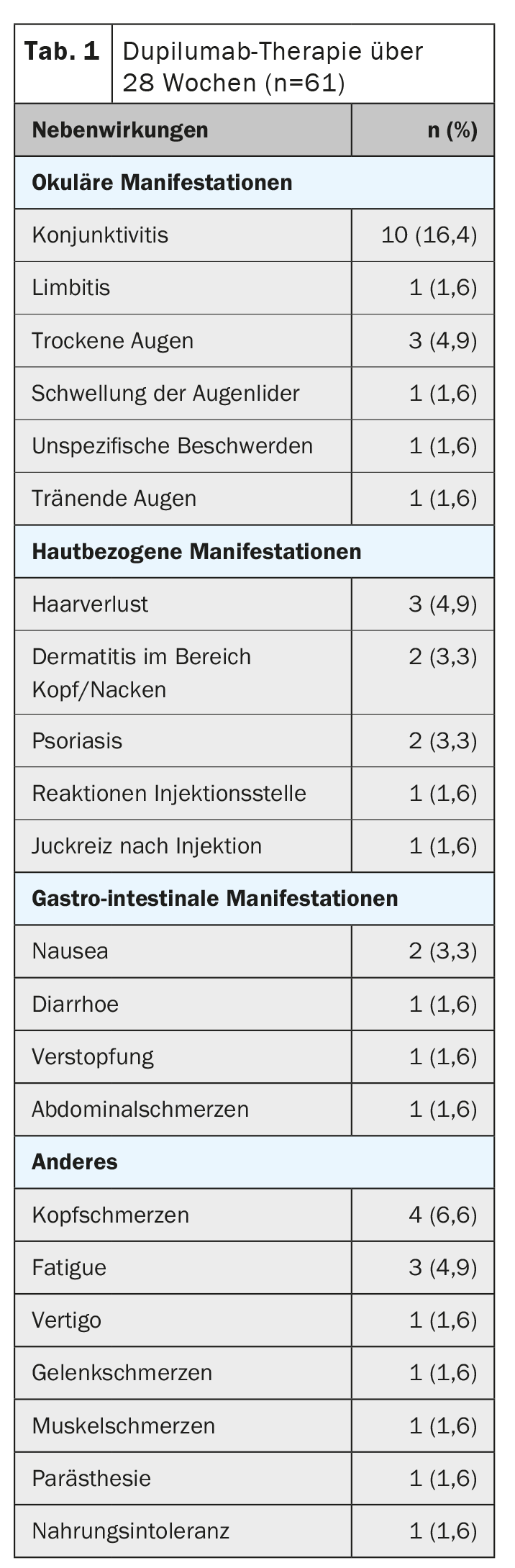
Of the 61 patients included, 75.4% and 49.2% achieved an EASI-50 and EASI-75 response, respectively (Fig. 1) [2]. The EASI-90 response rate was 24.6%. Lesion-free or nearly lesion-free according to the IGA score was 36.1%. An improvement of ≥4 points in POEM was achieved by 84.7%, whereas this proportion was 45.3% and 77.4% for NRS itch and NRS pain, respectively. Biomarkers TARC, PARC, periostin, sIL-2Ra, and eotaxin-3 decreased significantly during treatment. The p-EASI showed a significant correlation with disease severity. The most common adverse events were conjunctivitis (n=10) and headache (n=4) (Table 1).
Literature:
- Drug Information, www.swissmedicinfo.ch,(last accessed 04/13/2013).
- Kamphuis E, et al: Dupilumab in daily practice for the treatment of pediatric atopic dermatitis: 28-week clinical and biomarker results from the BioDay registry. Pediatr Allergy Immunol 2022; 33(12): e13887.
DERMATOLOGY PRACTICE 2023; 33(2): 52