The anti-IL-17A monoclonal antibody ixekizumab has already been approved since 2016 for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. In 2020, axial spondyloarthritis (axSpA) was added as another indication for which ixekizumab is a therapeutic option. Based on the COAST trial program, the efficacy and tolerability of ixekizumab in non-radiographic as well as radiographic axSpA in patients with or without pretreatment with TNF-α inhibitors wasproven.
The anti-IL-17A monoclonal antibody ixekizumab has already been approved since 2016 for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. In 2020, axial spondyloarthritis (axSpA) was added as another indication for which ixekizumab is a therapeutic option. Based on the COAST trial program, the efficacy and tolerability of ixekizumab in nonradiographic as well as radiographic axSpA in patients with or without pretreatment with TNF-α inhibitors has been demonstrated [1–4].
Psoriatic arthritis
Therapeutic strategies approved for psoriatic arthritis (PsA) include the use of NSAIDs, csDMARDs, biologics (TNF inhibitors, IL-12/23 and IL-17 inhibitors, T-cell modulators), and tsDMARDs (PDE4 inhibitors, JAK inhibitors). Which agents are used in which patients is determined individually according to the manifestation pattern and other factors (e.g., comorbidities, social factors, mode of application, and concomitant diseases).
With the American College of Rheumatology (ACR), the European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), therapy recommendations from three international institutions are available to provide guidance in prescribing [5]. As a first-line strategy in PsA treatment, the use of non-steroidal anti-inflammatory drugs (NSAIDs) is still recommended here, which can be used at symptom onset but also before diagnosis is confirmed. In everyday care, attention must be paid to substance-specific gastrointestinal, cardiovascular, and renal risks. Systemic glucocorticoids (GC), on the other hand, play only a minor role in PsA. However, intra-articular injection may be considered for the treatment of mono- or oligoarticular patterns of involvement or as an adjunct to existing therapy with disease-modifying antirheumatic therapies (DMARDs). DMARDs are used after a confirmed diagnosis has been made and if symptoms persist.
Significant improvement already after 6 weeks
Ixekizumab is indicated in the treatment of PsA in adults who have had an inadequate response to or are intolerant of one or more disease-modifying antirheumatic drugs (DMARDs), alone or in combination with methotrexate. This can lead to a significant improvement of skin florescences with an approximate normalization of the skin structure already after 6 weeks of therapy. In addition, a rapid reduction in itching and an improvement in quality of life have been described for the active ingredient.
Currently, pain improvement has been reported for ixekizumab in patients with and without measurable inflammation in psoriatic arthritis [6]. Having previously demonstrated the efficacy of ixekizumab (IXE) and adalimumab (ADA) in patients with PsA with reference to response rates at ACR50 and PASI(Psoriasis Area and Severity Index)-100 [7,8], efficacy of either IXE or ADA monotherapy on pain reduction beyond measurable inflammation in patients with active PsA and low C-reactive protein (CRP, <5 mg/l) at baseline was now evaluated.
The SPIRIT-H2H study included 95 monothera-py patients. Baseline characteristics were similar between the two treatment arms. In patients with persistent low inflammation (measured by CRP), there was no difference in improvement in joint pain between IXE- and ADA-treated patients. However, in patients with fluctuating inflammation, IXE-treated patients showed a numerically greater mean improvement in joint pain (VAS) vs. ADA-treated patients at week 16 (IXE: -31.64, ADA: -25.33). This held until week 52 (IXE: -47.69, ADA: -20.67). Significance in favor of IXE was measured at weeks 32 (p=0.0045) and 52 (p=0.0288). Similarly, numerically greater mean improvements in joint pain were measured in patients with sustained improvement in joint swelling as well as fluctuating improvement (assessed by SJC) in the IXE arm compared with ADA-treated patients from weeks 4 and 16, respectively, which were sustained through week 52.
This analysis suggests differential patterns of pain improvement in patients with low baseline CRP treated with IXE or ADA monotherapy, with a more favorable pain reduction outcome for IXE-treated patients. This is evident even when inflammation, as measured by CRP or SJC improvement, fluctuates. Thus, the study supports the hypothesis that IXE improves joint pain in PsA patients with and without measurable inflammation.
Axial spondyloarthritis
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease composed of non-radiologic axSpA and radiologic axSpA (r-axSpA). The latter, also known as ankylosing spondylitis (AS), is characterized by inflammatory back pain and radiological evidence of sacroiliac joint damage [9]. These manifestations, as well as peripheral musculoskeletal and extra-articular signs and symptoms, can contribute to mobility limitation, progressive disability, and decreased quality of life (QoL). Biologic disease-modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor inhibitors (TNFi) and an interleukin (IL)-17A antagonist, are recommended for the treatment of patients with axSpA who do not respond to or cannot tolerate nonsteroidal anti-inflammatory drugs (NSAIDs). However, up to 40% of patients do not achieve satisfactory disease control with TNF inhibitors [10], and treatment with TNFi may be contraindicated in certain patients.
The IL-17 pathway plays a key role in the pathogenesis of axSpA. Two randomized, controlled phase 3 clinical trials (COAST-V and COAST-W) evaluated the efficacy and safety of ixekizumab as a high-affinity monoclonal antibody selectively targeting IL-17A over a period of up to 52 weeks in patients with active radiographic axial spondyloarthritis (r-axSpA). Participants in COAST-V were bDMARD-naive, and those in COAST-W were TNF inhibitor-experienced.
To this end, in COAST-V, 341 patients with active r-axSpA were randomized 1:1:1:1 to 80 mg ixekizumab (IXE) every 2 or 4 weeks, placebo, or 40 mg adalimumab (ADA) biweekly. In COAST-W, 316 patients received 1:1:1 ixekizumab every 2 or 4 weeks or placebo. At week 16, patients receiving ixekizumab continued their assigned treatment; patients on placebo or ADA were switched 1:1 to IXE Q2W or IXE Q4W by week 52.
Good efficacy and safety
Both therapies with ixekizumab were shown to improve disease activity, physical function, objective markers of inflammation, quality of life, health status, and overall function over a period of up to 52 weeks.
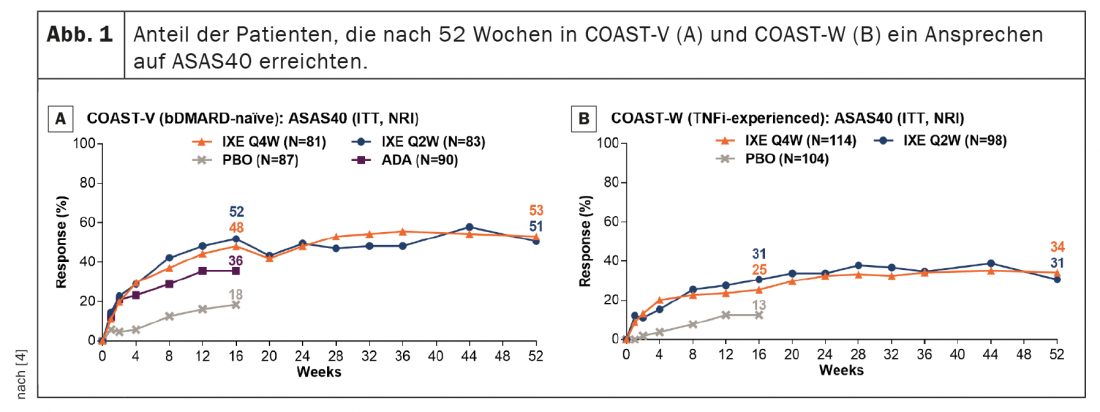
In patients continuously treated with ixekizumab, ASAS40 response rates persisted from week 16 to week 52 (Fig. 1) . ASAS40 response rates at week 52 were 53.1% (IXE Q4W) and 50.6% (IXE Q2W) in COAST-V and 34.2% (IXE Q4W) and 30.6% (IXE Q2W) in COAST-W. Patients randomized to placebo and re-randomized to ixekizumab at week 16 showed rapid improvement in ASAS40 response rates after switching to ixekizumab (Fig. 2); Response rates at week 52 (46.5% in COAST-V, 38.7% in COAST-W) were numerically similar to patients initially randomized to ixekizumab. In COAST-V, patients randomized to ADA showed further numerical improvements in ASAS40 response rates (36.0% at week 16, 51.2% at week 52) after switching to ixekizumab (Fig. 2A); response rates at week 52 were numerically similar to patients initially randomized to ixekizumab.
The pooled EAIR for psoriasis was 1.0. One patient had a serious adverse cerebrocardiovascular event (acute myocardial infarction) and two malignancies were reported (acute promyelocytic leukemia and bladder cancer). Fewer injection site reactions (ISRs) were reported with IXE Q4W (9.2%) than with IXE Q2W (17.2%). The number of patients reporting ISR decreased over time. Specifically, 6.4%, 3.8%, and 3.4% of patients under IXE Q4W and 14.3%, 8.6%, and 5.2% of patients under IXE Q2W reported ISR at weeks 0-12, weeks 12-24, and weeks 24-36, respectively. Few patients (IXE Q4W ≤1%; IXE Q2W approximately 3%) reported ISR beyond week 36.
In general, treatment responses were numerically lower in TNFi-experienced compared with bDMARD-naive patients, reflecting a more difficult-to-treat population with prior treatment failure and longer-standing disease [4]. Currently approved biologic therapies for axSpA include several TNFi and an IL-17A antagonist. Although only direct comparison studies can fully assess the relative efficacy and safety of different treatments, the ASAS40 results reported herein at week 52 are consistent with those reported for TNFi in patients who were bDMARD-naive and for secukinumab in subgroups of patients who were bDMARD-naive or who had previously failed TNFi treatment.
The discontinuation rate due to adverse events (AEs) was <4% in both studies, and serious adverse events (SUEs) were reported by <6% of patients. Most infections and ISRs were mild to moderate in severity. ISRs were more common in IXE Q2W than in IXE Q4W. In addition, ISRs were most commonly reported during the first 4 weeks of treatment and decreased in frequency over time. During the 52-week study periods of COAST-V and COAST-W (n=641), the pooled exposure-adjusted incidence rate per 100 patient-years (EAIR) of serious infections was 2.0 in patients treated with ixekizumab. Pooled EAIRs of Candida infection and grade 3/4 neutropenia were 1.0 and 0.2, respectively. Corresponding EAIRs for Crohn’s disease, ulcerative colitis, and inflammatory bowel disease (IBD) (NOS) were 0.8, 0.4, and 0.4, respectively (overall IBD EAIR: 1.6). The EAIR for anterior uveitis (AU) was 3.9; 15 of 20 patients (75%) had a history of AU, and 14 of 20 patients (70%) were from COAST-W. Fewer CED events were reported with IXE Q2W than with IXE Q4W, and there was no apparent association between duration of ixekizumab exposure and CED. Previous reports have shown that the EAIR for AU in patients with ankylosing spondylitis (AS) ranges from 2.6 to 3.5 for patients treated with TNFi [11].
In summary, the COAST-V and COAST-W data show that ixekizumab achieved sustained and clinically meaningful improvement in the signs and symptoms of active r-axSpA for up to 52 weeks. The safety findings were consistent with the known safety profile of ixekizumab. These results suggest that ixekizumab may be a treatment option for axSpA in patients who are bDMARD-naïve or who have previously had an inadequate response or intolerance to TNF inhibitors.
Study of the effect after remission
Results from randomized trials of tumor necrosis factor (TNF) inhibitor discontinuation in patients with axial spondyloarthritis suggest that discontinuation of TNFi leads to relapse in most patients and that continued treatment may be important for maintaining disease control. However, no studies to date have focused on the effects of continuation versus discontinuation of an interleukin (IL)-17A antagonist in patients with axSpA. COAST-Y is a follow-up study to COAST-V and COAST-W. The aim of this study, [12]published in 2021, was to fill this gap and investigate continuation vs. discontinuation of ixekizumab treatment in patients with axial spondyloarthritis who had achieved remission.
In the double-blind, placebo-controlled, randomized phase 3 extension study, patients who completed the original 52-week COAST-V, COAST-W, or COAST-X trials entered a 24-week introductory phase and continued either 80 mg IXE every 2 weeks (Q2W) or 4 weeks (Q4W). Patients who achieved remission (i.e., an Ankylosing Spondylitis Disease Activity Score (ASDAS) <1.3 at least once at Week 16 or Week 20 and <2.1 at both visits) were randomly assigned equally at Week 24 to continue IXE Q4W, IXE Q2W, or discontinue on placebo in a blinded fashion. The primary endpoint was the proportion of patients without relapse (ASDAS ≥2.1 at two consecutive visits or ASDAS >3.5 at each visit) after the 40-week randomised withdrawal-retreatment period (RWRP), with time to relapse being an important secondary endpoint.
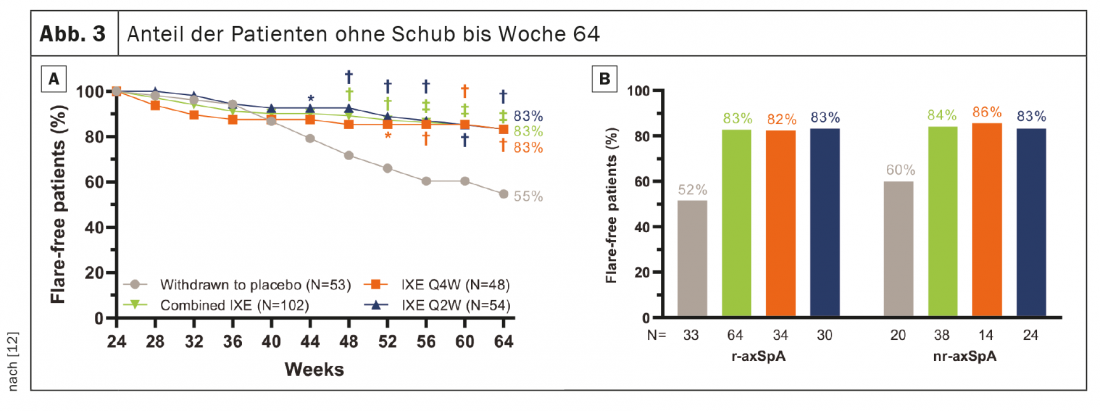
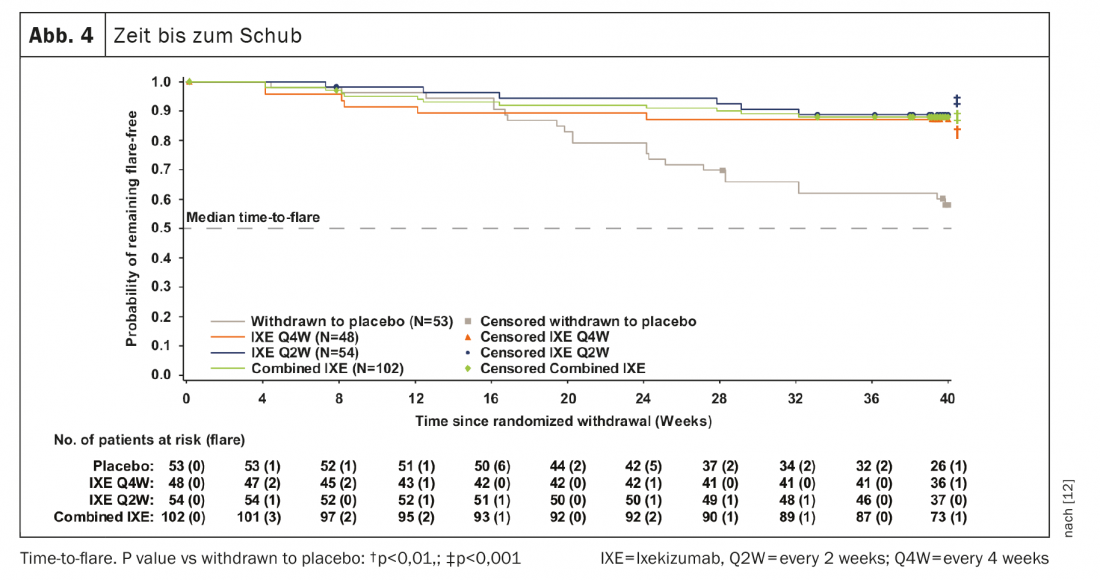
741 patients completed the 24-week introductory phase, and 155 entered RWRP. The primary and all major secondary goals were met at week 64. Forty weeks after randomized withdrawal, 83.3% of patients in the combined groups remained IXE (85/102, p<0.001), IXE Q4W (40/48, p=0.003) and IXE Q2W (45/54, p=0.001) relapse-free versus 54.7% in the placebo group (29/53). (Fig. 3A). The proportion of patients without relapse was similar between the patient subgroups with r-axSpA and nr-axSpA (Fig. 3B). Continuation of IXE significantly delayed time to relapse compared with placebo, with most patients remaining relapse-free for up to 20 weeks after discontinuation of IXE (Fig. 4). That 54.7% of patients who discontinued placebo remained relapse-free during RWRP was a higher value than in comparable discontinuation studies of adalimumab (47%) and certolizumab pegol (20.2%) [13–16].
Predictors and safety
Post-hoc analyses identified several patient characteristics associated with relapse, including ASDAS area under the curve, suggesting that patients with less well-controlled disease were more likely to have relapses over time than those whose disease control was stable. In addition, discontinuation of IXE, a higher baseline CRP level, and a nonnormal BMI (which was ≥25kg/m2 in most cases) were identified as associated with relapse. A higher BASDAI pain score at week 24 was also associated with relapse in patients who continued IXE treatment, but not in patients who discontinued on placebo.
The RWRP of COAST-Y included patients from across the axSpA spectrum with and without prior TNFi failure and with symptom duration ranging from 1.9 to 44.7 years (mean 12.7 years) [17]. There were no new or unexpected safety concerns during the RWRP. Treatment-emergent adverse events (TEAEs) were reported in 42.6% (IXE Q4W), 44.4% (IXE Q2W), and 52.8% (weaned to placebo) of patients and were generally mild to moderate in severity. Two patients (IXE Q2W) discontinued the study due to UE. SUEs were noted in two (4.3%) patients in the IXE Q4W group (benign ovarian germ cell teratoma and compression fracture), two (3.7%) patients in the IXE Q2W group (chronic tonsillitis and myelopathy in one patient and Clostridium difficile colitis in another), and one (1.9%) patient who had discontinued on placebo (soft tissue inflammation). Only one SUE (C. difficile colitis) resulted in discontinuation. There were no deaths and no reports of reactivation of tuberculosis, inflammatory bowel disease, serious adverse cardiovascular events (MACE), or malignancy.
Overall, these results suggest that ongoing IXE treatment is important for most patients to maintain long-term disease control [12]. However, the long duration of response to treatment after discontinuation of IXE suggests that temporary interruption of treatment, e.g., during infection or before surgical procedures, is unlikely to result in relapse in most patients. These results are important for clinicians when making treatment decisions regarding treatment interruption and optimizing the long-term management of axSpA.

Take-Home Messages
- Ixekizumab is approved for the treatment of moderate to severe plaque psoriasis, psoriatic arthritis and axial spondyloarthritis.
- In PsA, ixekizumab can lead to significant improvement in skin florescences after only 6 weeks of therapy. In addition, pain improvement has been reported topically in patients with and without measurable inflammation.
- In patients with active r-axSpA who are bDMARD-naive or TNFi-experienced, ixekizumab is an effective treatment.
- Ixekizumab can improve disease activity, physical function, objective markers of inflammation, quality of life, health status, and overall function in patients with r-axSpA for up to 52 weeks.
- In patients with axSpA in remission, continuous IXE treatment is associated with a higher likelihood of maintaining optimal disease control compared with IXE withdrawal.
Literature:
- Deodhar A, et al: Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020 Jan 4; 395 (10217): 53-64.
- van der Heijde D, et al: Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active controlled and placebo-controlled trial. Lancet 2018 Dec 8; 392 (10163): 2441-2451.
- Deodhar A, et al: Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results From a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients With Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol 2019; 71 (4): 599-611.
- Dougados M, Wei JCC, Landewé R, et al: Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomized, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis 2020; 79: 176-185; doi: 10.1136/annrheumdis-2019-216118.
- Köhm M, Burkhardt H, Behrens F: Therapeutic strategies of psoriatic arthritis. DMW – Deutsche Medizinische Wochenschrift 2020; 145(11): 773-780; doi: 10.1055/a-0964-0231.
- de Vlam K, Gallo G, Mease P, et al: Ixekizumab Shows a Pattern of Pain Improvement in Patients with and Without Measurable Inflammation in Psoriatic Arthritis. Arthritis Rheumatol 2021; 73 (suppl 10).
- Mease PJ, Smolen JS, Behrens F, The SPIRIT H2H study group et al: A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biologic-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020; 79: 123-131; doi: 10.1136/annrheumdis-2019-215386.
- Smolen JS, Sebba A, Ruderman EM, et al: Efficacy and Safety of Ixekizumab with or Without Methotrexate in Biologic-Naïve Patients with Psoriatic Arthritis: 52-Week Results from SPIRIT-H2H Study. Rheumatol Ther 2020; 7: 1021-1035; doi: 10.1007/s40744-020-00250-3.
- Sieper J, Poddubnyy D: Axial spondyloarthritis. Lancet 2017; 390: 73-84; doi: 10.1016/S0140-6736(16)31591-4.
- Sepriano A, Regel A, van der Heijde D, et al: Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 2017; 3: e000396; doi: 10.1136/rmdopen-2016-000396.
- Deodhar A, Miceli-Richard C, Baraliakos X, et al: Low incidence of both new-onset and flares of uveitis in secukinumab-treated patients with ankylosing spondylitis: clinical trial and post-marketing safety analysis. Ann Rheum Dis 2018; 77(Suppl 2): 999; doi: 10.1136/annrheumdis-2018-eular.4474.
- Landewé RBM, Gensler LS, Poddubnyy D, et al: Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y). Ann Rheum Dis 2021; 80: 1022-1030; doi: 10.1136/annrheumdis-2020-219717.
- Landewé RB, van der Heijde D, Dougados M, et al: Maintenance of clinical remission in early axial spondyloarthritis following certolizumab pegol dose reduction. Ann Rheum Dis 2020; 79: 920-928; doi: 10.1136/annrheumdis-2019-216839.
- Landewé R, Sieper J, Mease P, et al: Efficacy and safety of continuing versus withdrawing adalimumab therapy in maintaining remission in patients with non-radiographic axial spondyloarthritis (ABILITY-3): a multicentre, randomised, double-blind study. Lancet 2018; 392: 134-144; doi: 10.1016/S0140-6736(18)31362-X.
- Haibel H, Heldmann F, Braun J, et al: Long-term efficacy of adalimumab after drug withdrawal and retreatment in patients with active non-radiographically evident axial spondyloarthritis who experience a flare. Arthritis Rheum 2013; 65: 2211-2213; doi: 10.1002/art.38014.
- Song IH, Althoff CE, Haibel H, et al: Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 2012; 71: 1212-1215; doi: 10.1136/annrheumdis-2011-201010.
- Smolen JS, Schöls M, Braun J, et al: Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018; 77: 3-17; doi: 10.1136/annrheumdis-2017-211734.
InFo PAIN & GERIATURE 2021; 3(2): 14-18.