The treatment of sleep disorders is a common and sometimes intractable problem in medical practice. The treatment starts with finding and avoiding disturbing factors. If this does not lead to the desired success, most doctors try to achieve the desired improvement by prescribing a well-tolerated sleep medication. Tolerability is paramount in the choice of a sleep medication because many of these patients are also taking other medications and the benefit balance, if successful, favors tolerable medications.
Patients and method
The participating 29 physicians agreed on a structured patient form with the usual parameters for an observational study (AWB) of sleep medication. Before starting, a neutral evaluation site was designated (Heinrich Medical, 8913 Ottenbach ZH), and the anonymously completed patient sheets were handed over to the evaluation site. The results were summed and percentages were calculated.
- A total of 408 patients, aged 10-97 years, were enrolled in the observational study.
- Twelve patients did not show up for the follow-up appointment and the physicians were unable to determine any results of the application.
- At least one follow-up visit or survey occurred in 396 patients. The mean observation period was 20 days.
Results
- The efficacy of Similasan® Sleep Disorder Tablets resulted in a very good or good overall rating in 77% of patients. Only 23% found the effectiveness insufficient.
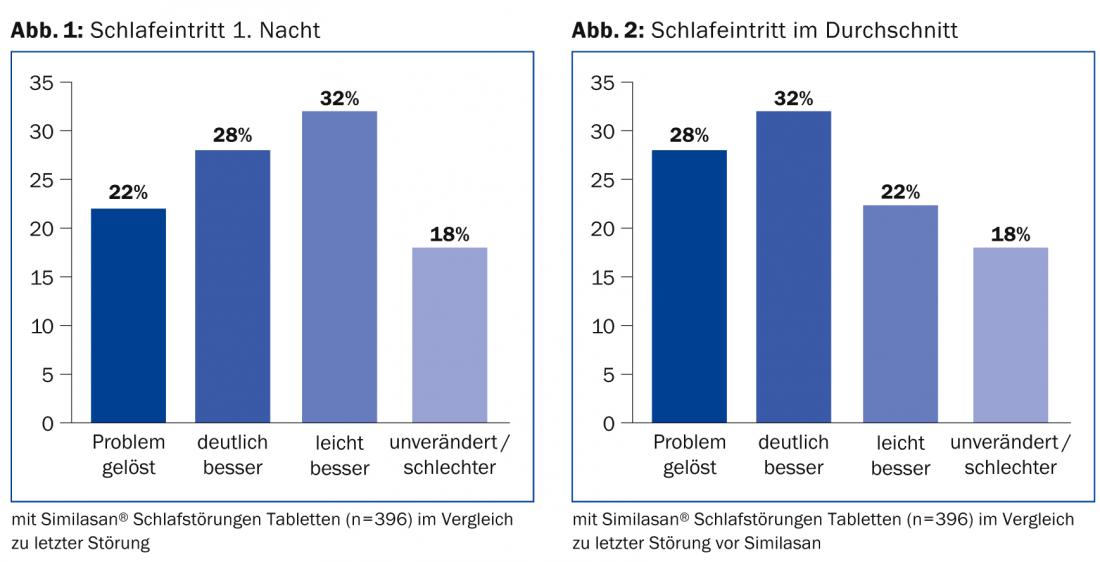
- Sleep onset improved in 82% of patients (Figs. 1 and 2) . For the most part, the improvement was already achieved from the first night of the application observation (Fig. 1).
- In the majority of patients, efficacy remained the same as on the first day throughout the observation period. For some, the level of efficacy improved and for very few, it worsened, resulting (on average across treatments) in the results shown in Figure 2.
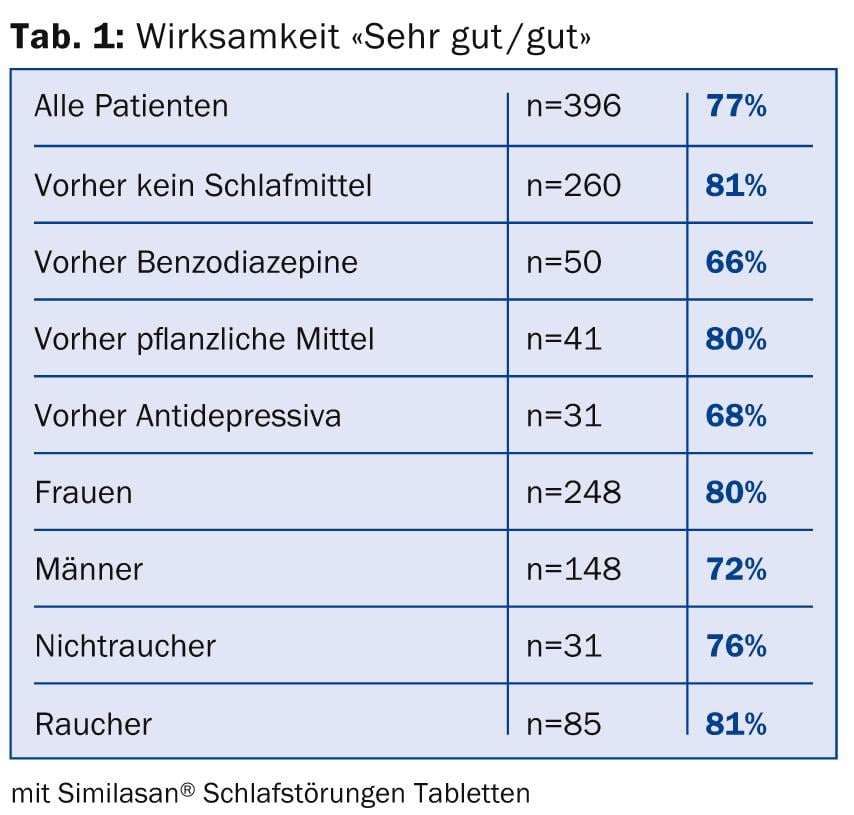
- Demographic differences were several. They are most evident in the overall assessment of effectiveness . In this large patient population, one can see which patient groups benefited more than average from the treatment and which had below-average efficacy (Table 1).
- Available experience in medical practice shows that patients without prior sleep-promoting medication benefit more than average from Similasan® Sleep Disorder Tablets, with 85% improvement and 81% very good or good effect. Patients previously treated with herbal sleep aids had similarly good results (80% good and very good assessment). As expected, efficacy was lower, although still respectable, in patients previously treated with benzodiazepines or antidepressants (66% and 68% very good and good effect, respectively). Significantly above-average overall ratings of effect were among smokers (81%, n=85), persons younger than 45 years (82%, n=110), and above-average were among women (80%, n=248). Overweight individuals (over 81 kg) had significantly worse outcomes, with only 68% very good and good overall ratings (n=91).
Compatibility
- Tolerance was good and problem-free in 98% of patients. Overall, 97% of patients had no side effects.
- Side effects were reported by 3% of patients. These were all unproblematic and the relationship to treatment was questionable in most cases. One patient reported severe daytime sleepiness, and one patient had an increase in preexisting pericarditis symptoms. All other side effects were mild or moderate in nature and did not cause any problems. One-third of patients with side effects continued treatment.)
High patient satisfaction
- The positive overall assessments of efficacy and tolerability indicate that most patients were satisfied. This is confirmed by the high number of patients who wanted to continue treatment at the end of AWB (72%).
- Together with patients who no longer required treatment at the end of observation (3%), 75% of patients in this AWB had sustained satisfaction or relief from sleep problems.
Conclusion
This AWB in 396 patients impressively confirmed the efficacy and tolerability of an average of 1.7 Similasan® sleep disorder tablets per night. Sleep quality was rapidly and significantly improved in most patients, avoiding the use of sleeping pills with higher side effect potential.
The tolerance of this homeopathic medication did not lead to any problems and according to the prescription information no problematic side effects are to be expected. Similasan® Sleep Disorder Tablets are consequently very suitable for the treatment of sleep problems when avoiding the disturbing causes alone is not enough.
Simon Feldhaus, MD
Ethical requirements
The data collection of this observational study was approved by the cantonal ethics committee prior to initiation.
Conflicts of Interest
There are no conflicts of interest. Similasan AG (manufacturer) has agreed to compensate the effective time spent on application observation with a Tarmed-compliant fee. However, participating physicians were completely free to include patients and assess observations. The evaluation is based solely on the arithmetical additions and percentages of the results of the evaluable patient forms.
HAUSARZT PRAXIS 2014; 9(2): 36-37











