Type 2 diabetes (T2D) is a progressive disease characterized by a deterioration in the function of pancreatic β-cells and increased insulin resistance. Although there are many diabetes medications on the market, there are still a high number of patients who are unable to reach their glycemic goals.
Dr. Yun Kyung Cho, Assistant Professor at Asan Medical Centre in Seoul, South Korea, and her colleagues conducted a prospective, multicentre, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety profile of pioglitazone (PIO) compared to placebo (PBO) in patients with type 2 diabetes who were inadequately controlled with metformin and dapagliflozin. The primary endpoint of the study was the mean change in HbA1c levels over a 24-week period.
The Phase 3 study was conducted from May 2021 to March 2023 in 42 centers in Korea. A total of 366 patients with HbA1c levels between 7.0% and 10.5% were included in the study, although they were treated with metformin and dapagliflozin. Participants were randomly allocated in a 1:1:1 ratio to receive either placebo (PBO), 15 mg pioglitazone daily (PIO15) or 30 mg pioglitazone daily (PIO30).
PIO15 and PIO30 significantly reduced HbA1c levels
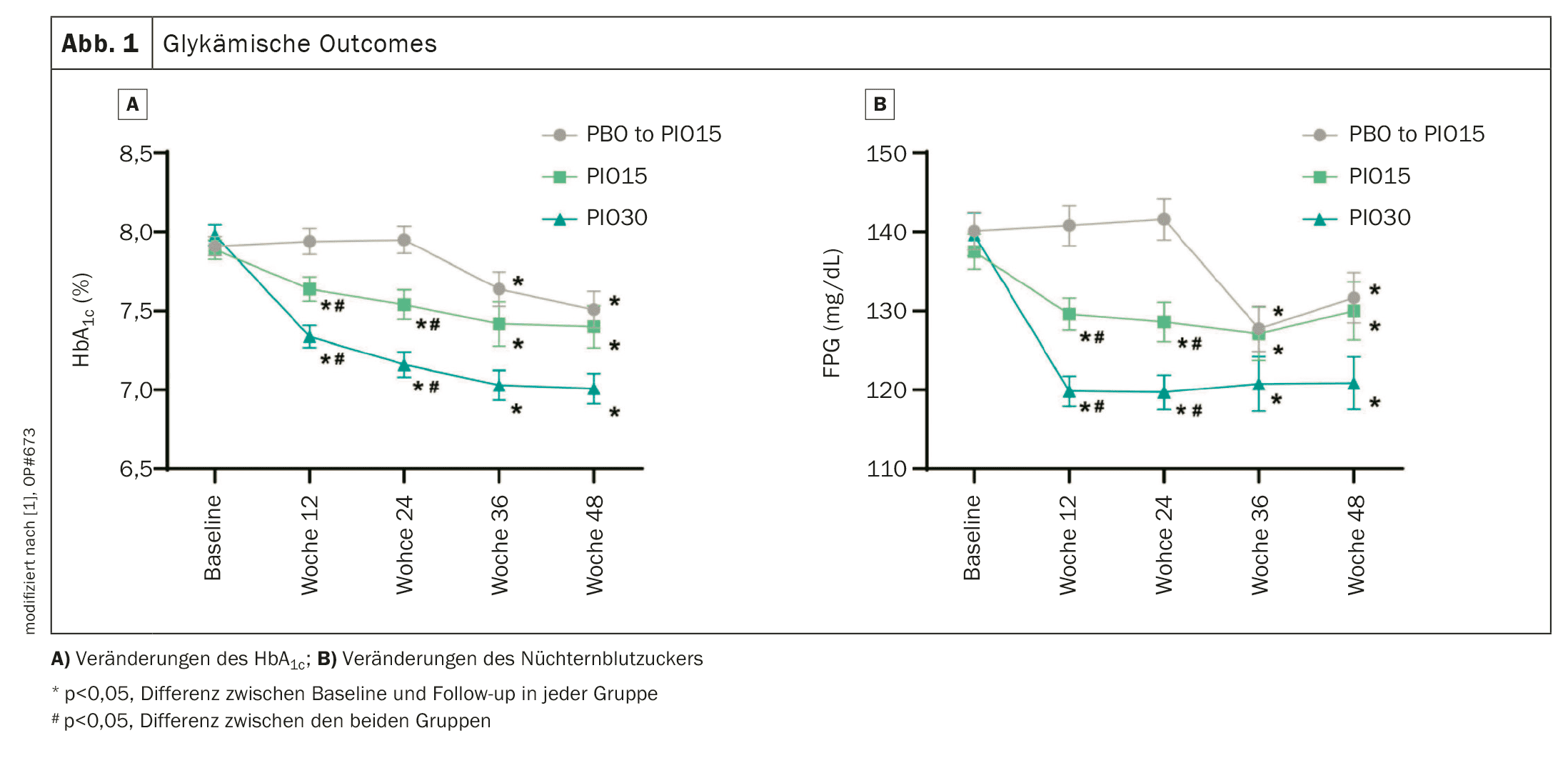
The mean age of the 366 participants (PBO: n=124, PIO15: n=118, PIO30: n=124) was 55.6 years, the mean duration of diabetes was 8.7 years, with a baseline HbA1c of 7.9%. At 24 weeks, HbA1c levels were significantly lower in the PIO15 and PIO30 groups compared to baseline, with between-group differences of -0.38% and -0.83%, respectively, compared to the PBO group.
The fasting plasma glucose (FPG) level was significantly reduced in the PIO15 (–8.32 mg/dl) and PIO30 (–19.86 mg/dl) groups. The proportion of patients achieving the HbA1c target of <7.0% at week 24 was 12.9%, 31.4% and 52.0% in the PBO, PIO15 and PIO30 groups, respectively (Fig. 1). Both PIO cohorts achieved a significantly higher rate of HbA1c targets than the PBO arm. At a glycemic target of <6.5%, the PIO30 group had a higher percentage of participants reaching glycemic target than the PBO group (18.6% vs. 3.2%).
PIO reduced insulin resistance and showed a favorable safety profile
The results showed that PIO improved insulin sensitivity in patients with T2D receiving metformin and dapagliflozin. Insulin sensitivity is measured by the homeostatic model assessment of insulin resistance (HOMA-IR). Administration of PIO15 and PIO30 significantly lowered HOMA-IR values and preserved HOMA-β values (insulin secretion, homeostatic model assessment of β-cell function, HOMA-β), which is different from the deterioration observed in the PBO group.
Although PIO is associated with weight gain, body weight in this study increased by only 1 kg (PIO15) or 2 kg (PIO30) when PIO was co-administered with metformin or dapagliflozin, Dr. Cho explained. Rates of adverse events did not differ significantly between the groups, indicating a favorable safety profile for the triple combination therapy of metformin, dapagliflozin and pioglitazone. The rates of edema and weight gain were slightly higher in the PIO groups compared to those in the PBO arm. The other adverse events were comparable in the PBO, PIO15 and PIO30 groups.
The results showed that the addition of pioglitazone to metformin and dapagliflozin is a safe and effective strategy to further improve glycemic control in patients with type 2 diabetes, the speaker concluded. The use of 30 mg pioglitazone resulted in a superior reduction in HbA1c and fasting plasma glucose with a favorable safety profile, making it a viable third-line therapy for patients who are inadequately controlled with a dual therapy of metformin and dapagliflozin.
Source:
- Cho YK: Efficacy and safety of pioglitazone add-on in patients with type 2 diabetes inadequately controlled with metformin and dapagliflozin: a randomized, double-blind, and placebo-controlled study. Oral Presentation #673, Session SO 053: Oral agents to control glucose metabolism. EASD, 12.09.2024.
InFo DIABETOLOGIE & ENDOKRINOLOGIE 2024; 1(4): 21–22 (published on 29.11.24, ahead of print)