The efficacy of Ginkgobiloba extract EGb 761 in cognitive disorders such as dementia is always doubted. A meta-analysis recently evaluated seven clinical trials with a high qualitative level and again demonstrated the efficacy of EGb 761 in dementia.
The aim of the meta-analysis was to investigate the efficacy and safety of Ginkgo biloba extract EGb 761 in dementia, incorporating the most recent data [1]. EGb 761 is a dry extract of Ginkgo biloba leaves with a drug-extract ratio of 35-67:1 and acetone (60%) as the extracting agent. The extract is adjusted to 22-27% flavone glycosides and 5-7% terpene lactones, calculated as 2.8-3.4% ginkgolides A, B, C and 2.6-3.2% bilobalides. The content of ginkgolic acids must not exceed 5 ppm.
Studies
RCT studies that met the following criteria were included for the review: at least 20 weeks of study duration, subjects diagnosed with dementia (AD, VaD, or mixed) according to ICD-10, and at least two typical primary outcome variables for dementia assessment (cognitive function, activities of daily living, and overall clinical assessment). Studies that used EGb 761 as an add-on treatment to cholinesterase inhibitors were excluded.
Seven studies met these criteria, and their JADAD score was 3-5 [2–10]. The two studies by Kanowski [4,5] and by Le Bars [6,7] are each the same study that was later reevaluated for subgroup analysis.
Six studies enrolled patients with neuropsychiatric symptoms (NPS) [2–9]. In four of these studies [2,3,8,9], the presence of NPS was a mandatory inclusion criterion. In these studies, a total of 2684 patients were observed, 1423 in the EGb 761 groups, 1261 in the placebo groups. In the studies by Le Bars and by Schneider, the daily dose of EGb 761 was 120 mg; in the other studies, it was 240 mg.
Target parameter
Cognitive functions: All studies evaluated cognitive functions. Five studies [2–4,8,9] used the Syndrome Short Cognitive Performance Test (“SKT”), and two studies [6,10] tested cognitive function with the Alzheimer’s Disease Assessment Scale (ADAS-cog). The two tests have a great correspondence, test the same abilities and are therefore comparable.
Daily activities: All studies reviewed the subjects’ daily activities. Two studies [6,10] used the Geriatric Evaluation by Relative’s Rating Instrument (GERRI) for this purpose, and two studies [8,9] used the Gottfries-Bråne Standing Scale Activities of Daily Living Test (GBS-ADL). Two studies [3,4] assessed activities of daily living with the Alzheimer’s Disease Activities of Daily Living International Scale (ADL-IS), and one study [5] used the Nuremberg Aging Observation Scale (NAB).
Overall clinical assessment: all seven studies reviewed conducted an overall clinical assessment (CGI). Two studies [4,6] did so with the Traditional Clinical Global Impression of Change (CGIC), three studies [2,3,10] with the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change (ADCS-CGIC), and two studies [8,9] with the Gottfries-Bråne Standing Scale, a geriatric global assessment.
Results
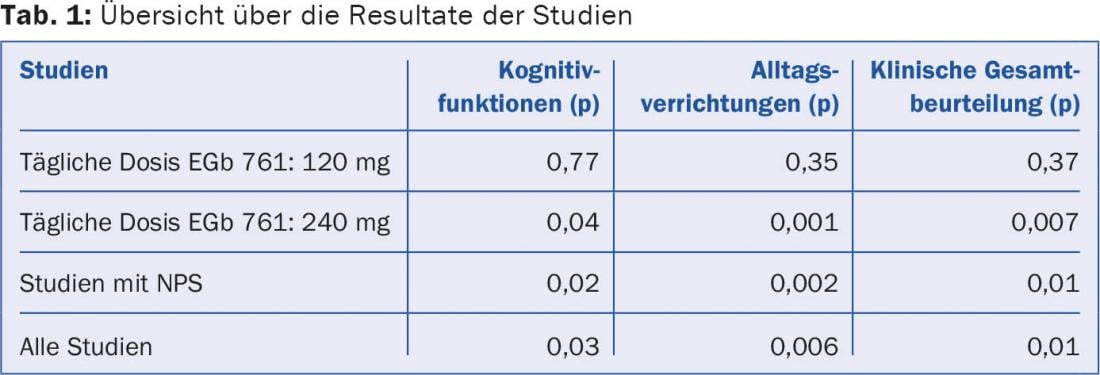
Cognitive functions: Five of the seven studies [2–5,8,9] showed significant improvement for the EGb 761 group compared to placebo (p=0.02), while two studies [6,7,10] showed non-significant improvement for the EGb 761 group (p=0.77). In the meta-analysis of all seven studies, EGb 761 was found to be significantly superior to placebo (p=0.03). A subgroup analysis of studies evaluating cognitive function with SKT showed an even more significant superiority of EGb 761 (p=0.02). The effect of EGb 761 on cognitive functions is dose-dependent. The two studies with a daily dose of 120 mg showed non-significant superiority, whereas the superiority of the studies with 240 mg EGb 761 per day was significant. The greatest improvement was seen in those studies that included NPS (p=0.02).
activities of daily living: Meta-analysis showed significant superiority for EGb 761 groups compared to placebo (p=0.006). Again, the studies with a daily dose of 240 mg proved significantly superior to the studies with only 120 mg: Only they reached significance (p=0.001 vs. p=0.35). Those studies with NPS performed best compared to placebo (p=0.002).
Overall clinical assessment: The same picture was seen in the overall clinical assessment: the meta-analysis of all seven studies showed significant superiority for EGb 761 over placebo (p=0.01). The two studies with a daily dose of 120 mg did not reach significance (p=0.37). However, the EGb 761 groups with a daily dose of 240 mg were found to be significantly superior to placebo (p=0.007). The studies with NPS again demonstrated a significant benefit over placebo. Table 1 shows an overview of the results.
Adverse events
In two of the seven studies, the number of subjects with at least one documented adverse event was slightly higher in the EGb 761 group than in the placebo group. No difference was found in the other studies. Overall, no trend for increased risk of adverse events was evident in the verum group. The most common documented adverse events were related to headache (EGb 761 11% vs. placebo 14%), dizziness (5% vs. 9%), hypertension (4% vs. 4%), tinnitus (3% vs. 4%), angina (3% vs. 4%), and respiratory infections (3% vs. 3%).
Summary
This meta-analysis of high-quality studies that reviewed the efficacy and safety of Ginkgo biloba extract EGb 761 versus placebo in dementia with or without neuropsychiatric symptoms showed significant superiority of EGb 761 over placebo. This involved cognitive function, activities of daily living, and overall clinical assessment. While the studies with a daily dose of 120 mg EGb 761 documented nonsignificant superiority, the daily dose of 2× 120 mg proved significantly superior. EGb 761 is particularly effective in the presence of neuropsychiatric symptoms. In all studies including NPS, EGb 761 showed significant superiority.
No significant differences in documented adverse events were found between the verum and placebo groups in terms of type and frequency. Thus, EGb 761 offers itself as an effective and safe therapy for dementia with or without NPS.
Literature:
- Gauthier S, Schlaefke S: Clinical interventions in aging 2014; 9: 2065-2077.
- Herrschaft H, et al: J Psychiatr Res 2012; 46(6): 716-723.
- Ihl R, et al: Psychiatry 2011; 26(11): 1186-1194.
- Kanowski S, et al: Pharmacopsychiatry 1996; 29(2): 47-56.
- Kanowski S, Hoerr R: Phyrmacopsychiatry 2003; 36(6): 297-303.
- Le Bars PL, et al: JAMA 1997; 278(16): 1327-1332.
- Le Bars PL, et al: Dement Geriatr Cogn Disord 2000; 11(4): 230-237.
- Napryeyenko O, Borzenko I: Drug Research 2007; 57(1): 4-11.
- Nikolova S, et al: Bulgarian Neurology 2013; 14(3): 139-143.
- Schneider LS, et al: Curr Alzheimer Res 2005; 2(5): 541-551.
HAUSARZT PRAXIS 2015; 10(8): 2











