At the first Swiss symposium on peritoneal malignancies and HIPEC in St. Gallen, experts spoke on the topics of ovarian cancer and metastatic colorectal cancer. It was about current and potential future treatment options. What is the place value of bevacizumab? Where are the opportunities for individualized therapy? And what are confirmed predictive markers that should guide treatment decisions?
The same principles apply to the oncological treatment of ovarian carcinoma as to tubal and peritoneal carcinomas. According to Prof. Beat Thürlimann, MD, of the Cantonal Hospital of St. Gallen, there are well-established standards, but hardly any gold standards. Such are:
- Optimal surgery with debulking
- First-line chemotherapy: platinum-based
- Second-line chemotherapy: platinum-based combination therapy.
- Palliative surgery.
Relatively new trends include bevacizumab after primary debulking as an adjunct to chemotherapy (available) and cediranib and olaparib (soon to be available) in platinum-sensitive progressive forms.

Bevacizumab (anti-VEGF): The ICON7 study (phase III) evaluated the safety and efficacy of bevacizumab as an adjunct to standard chemotherapy with carboplatin and paclitaxel in 1528 women with recently diagnosed ovarian cancer (FIGO stage I-IIA [klarzellig oder Grad 3] or FIGO stage IIB-IV). After the primary analysis [1] of progression-free survival had already demonstrated a benefit of the addition, especially in those women at high risk for progression, the final results regarding overall survival were presented at the ECC Congress 2013: While in the overall population no significant difference between control and study group could be shown, the subgroup with high risk for progression (suboptimal “debulked” stage III, stage IV and non-operated patients) benefited particularly strongly: Here, overall survival improved significantly.
The AURELIA trial was the first randomized trial to analyze the addition of bevacizumab to chemotherapy in a platinum-resistant population with ovarian cancer. The participants had already undergone two or more cancer therapies. They were randomized to receive either chemotherapy (paclitaxel, topotecan, “pegylated liposomal doxorubicin” [PLD]) or bevacizumab plus chemotherapy, until progression or unsustainable toxicities. As a result, the addition of bevacizumab was shown to significantly prolong progression-free survival by approximately three months. In contrast, the benefit in overall survival (also about three months) did not reach statistical significance, as illustrated by another presentation at the ECC Congress 2013.
Cediranib (anti-VEGF): The ICON6 study (phase III) evaluated cediranib in the treatment of platinum-sensitive, relapsed ovarian cancer. Accordingly, participants had already undergone first-line platinum-based chemotherapy and, after recurrence, were taking either chemotherapy alone (arm A) or chemotherapy plus cediranib (arms B and C). In addition, a maintenance phase with cediranib was given in the third study group (Arm C) and in the other two (Arm A and B) one initiated with placebo.
Chemotherapy plus cediranib followed by cediranib maintenance was shown to provide a significant survival benefit for this population (compared with chemotherapy alone). Furthermore, there is strong evidence for an effect of cediranib on progression-free survival during and after chemotherapy.
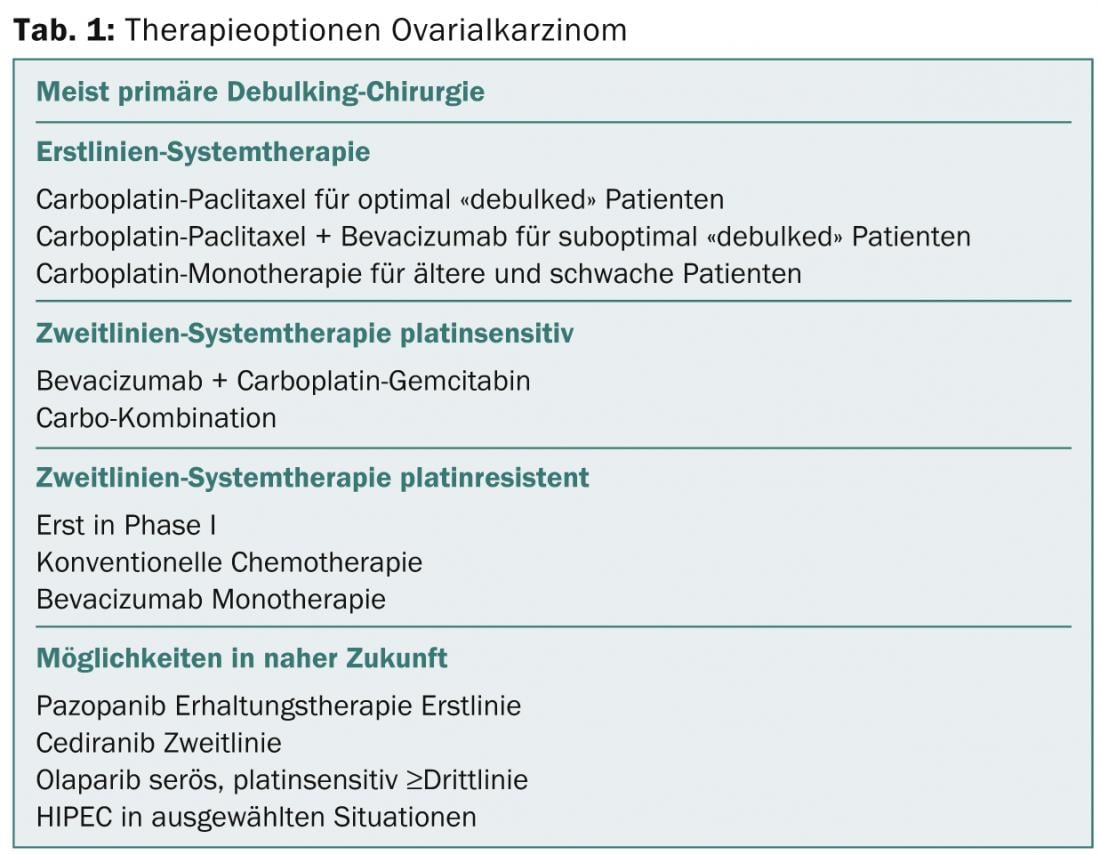
“So there are many options for the treatment of primary ovarian cancer. The therapy options are increasing, but ultimately it is always a question of cost,” concludes Prof. Thürlimann. Table 1 provides an overview.
Peritoneal metastatic CRC.
Progress in metastatic colorectal cancer (mCRC) is largely due to more aggressive surgery, better systemic therapy, and closer collaboration between oncology and surgery.
Prof. Dr. med. Ulrich Güller, Cantonal Hospital St. Gallen, first discussed systemic treatment: “Conventional chemotherapy is successfully carried out today as mono- or combination treatment with the help of the active ingredients 5-fluorouracil (5-FU), capecitabine (oral 5-FU preparation), irinotecan and oxaliplatin. However, we always need new targets, and we will only find them if we learn to understand tumor cell proliferation better and in more detail.”
For example, a new drug that has shown survival benefit in a phase III trial as second-line therapy is aflibercept [2]. It blocks the activity of three pro-angiogenic growth factors: VEGF-A, VEGF-B and the so-called placental growth factor (PIGF). It prevents these growth factors from binding to the cancer cells’ receptors, thus suppressing the growth of new blood vessels.
Antibodies used in mCRC nowadays are bevacizumab (anti-VEGF-A), cetuximab (anti-EGFR), and panitumumab (anti-EGFR). Several studies showed that these agents can improve response rates and survival.

In addition, regorafenib [3], an oral multikinase inhibitor with intracellular activity, has been approved. Specifically, it blocks several kinases involved in tumor angiogenesis/oncogenesis (including VEGFR1, VEGFR2, VEGFR3, KIT, RET, PDGF).
“So this is our situation today: We have conventional chemotherapy, aflibercept, three antibodies and a tyrosine kinase inhibitor,” Prof. Güller explained. “In the coming years, BRAF, PIK3, MEK and mTOR inhibitors are expected to gain importance in mCRC. In addition, the goal in the future is to administer treatment tailored to the individual patient,” Prof. Güller opined. “Determination of RAS status (KRAS/NRAS) is a first step towards such individualized therapy: it has been shown that only patients with RAS wild-type benefit from EGFR antibodies [4, 5]. Thus, determination of RAS status is necessary in any case before starting treatment with an EGFR antibody, as RAS status has a clear predictive value.”
This was most recently confirmed by the FIRE-3 trial, in which, in a post-hoc analysis, the overall survival benefit of adding cetuximab to FOLFIRI (as opposed to bevacizumab/FOLFIRI) was still significantly greater for RAS wild-type patients compared to the original KRAS wild-type population.
Source: 1st Swiss Symposium Peritoneal Malignancies and HIPEC, January 23, 2014, St. Gallen.
Literature:
- Perren TJ, et al: A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011 Dec 29; 365(26): 2484-2496. doi: 10.1056/NEJMoa1103799.
- Van Cutsem E, et al: Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012 Oct 1; 30(28): 3499-3506. epub 2012 Sep 4.
- Grothey A, et al: Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013 Jan 26; 381(9863): 303-312. doi: 10.1016/S0140-6736(12)61900-X. Epub 2012 Nov 22.
- Van Cutsem E, et al: Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011 May 20; 29(15): 2011-2019. doi: 10.1200/JCO.2010.33.5091. epub 2011 Apr 18.
- Douillard JY, et al: Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013 Sep 12; 369(11): 1023-1034. doi: 10.1056/NEJMoa1305275.
InFo Oncology & Hematology 2014; 2(2): 18-21.