As a serous layer, the pleura covers the thoracic organs and the inner skeletal side to the thoracic cavity. The small normal amount of pleural fluid responsible for non-irritant pleural sliding is both produced and reabsorbed via lymphatic pathways primarily in the parietal pleura.
As a serous layer, the pleura covers the thoracic organs and the inner skeletal side to the thoracic cavity. The physiologic negative pressure in the pleural cavity to maintain positive transpulmonary pressure is -3-5 cm H2O. The small normal amount of pleural fluid responsible for non-irritant pleural sliding is both produced and reabsorbed via lymphatic channels mainly in the parietal pleura. This capacity can increase by a factor of up to 20 depending on demand, so a relevant pleural effusion only occurs with a significant increase in the amount of fluid produced or reduced resorption capacity. For this purpose, the diaphragm has the function of a pump, which also contributes to pleural homeostasis.
Ultrasound examination of the thorax and pleura should ideally be performed in the sitting position. Sonographically, about 70% of the pleura can then be seen, and the parts of the pleura hidden by ribs can often still be seen by tilting the transducer. To obtain an overview, the examination should be started with a convex transducer (3-5 MHz); for a more detailed assessment of the structures near the transducer on the thoracic wall, the additional use of a higher-frequency linear transducer may then be useful. Sound waves are reflected from interfaces with a high impedance difference, so the surface of the ribs as well as the visceral pleura provide a natural barrier to ultrasound imaging.
Pleurasono as the most sensitive detection method
The detection of fluid in the pleural cavity was one of the first and remains one of the most important issues in clinical ultrasonography. Even the smallest amounts of fluid can be detected sonographically – much earlier than in computer tomography or even in X-rays. Various simple formulas exist for quantifying the amount of effluent, which can be particularly important for progress controls. The reproducibility of the examination and transducer position is important for this. In clinical practice, for example, a simple measurement of the subpulmonary effusion height, i.e., the distance between the lower edge of the lung and the diaphragm, is well practicable: thus it is possible to distinguish between low (<2 cm), moderate (2-5 cm), and large (>5 cm) effusion. With increasing examination experience, this is a sufficient procedure for clinical routine, since the absolute effusion volume is usually not important. All volume estimation formulas can only be applied to freely draining and not to encapsulated or septated pleural effusions.
Pleural effusions can have a variety of causes. The ultrasound image of the effusion alone often does not provide sufficient information to establish the cause of the effusion. However, sonography offers significant advantages, particularly over computed tomography:
- Radiation freedom,
- a significantly better representation of delicate solid structures in the effusion,
- as a real-time method, additionally provides a dynamic assessment of lung sliding as well as ventilation and blood flow.
Cause of atelectasis can often be differentiated sonographically
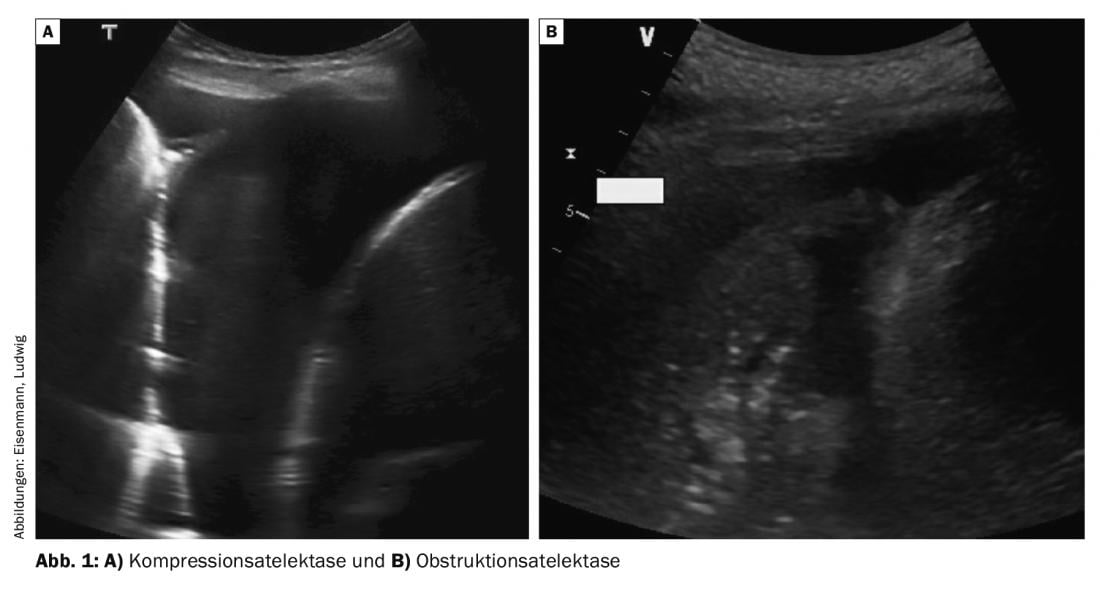
Larger effusions cause caudal displacement of the diaphragm and the adjacent lung – initially the lower lobe – is compressed. This homogeneous compression atelectasis moves respiratory and also pulse synchronously in the effusion (so-called “waving finger”) and can often be partially re-aerated during deep inspiration (Fig. 1A) . In dyspnea, therapeutic thoracentesis in this situation can achieve rapid expansion and thus symptom relief. In contrast, central airway obstruction (e.g., due to tumor, secretion, or foreign body) presents with obstructive atelectasis, which is less compressed and hardly mobile (Fig. 1B). Often, fluid-filled bronchi due to secretion retention can also be visualized (fluidobronchogram). Pleural effusion is usually less in obstructive atelectasis, and therapeutic effusion puncture to relieve dyspnea is not effective unless the bronchial cause is also corrected.
Additional sonographic evidence of effusion genesis can be obtained from concomitant findings in adjacent structures (Table 1).
Pleural puncture under sonographic view
Each pleural effusion should be punctured once diagnostically. In the case of bilateral effusions, this should be performed laterally and, if necessary, bilaterally, since different causes may well be present. Effusion punctures and drainage installations are performed at the upper rib margin. Any aberrant vasculature should be excluded by sonography prior to puncture. This usually reliably avoids injury to the intercostal vessels and nerve cords.
Puncture monitoring can be performed – analogous to vascular puncture, for example – by continuous visualization of the inserted puncture instrument; in the event of possible contact of the puncture instrument with the transducer, unconditional sterility must be ensured. In contrast, sonographic marking of the puncture site is also sufficient for large volumes of fluid.
For effusion assessment, a distinction is made between a transudate and an exudate based on the paraclinical effusion profile using the Light criteria. The essential parameters here are the serum/effluent ratios of protein, glucose and LDH. An unclear pleural effusion must also be examined microbiologically, but this often does not allow reliable determination of the pathogen. Improvement in sensitivity is often achieved by an additional biopsy of the parietal pleura for microbiologic material observation.
To avoid clinically significant re-expansion edema, limiting the amount of puncture possible in a single session to 1-1.5 liters is common, although the data do not provide compelling evidence in this regard. For larger effusions, fractional drainage via a thin-lumen catheter is therefore an option, so that a repeat puncture trauma can be avoided. This can be placed using the Seldinger technique, for example.
After the puncture, imaging control is always required to rule out pneumothorax. The use of sonography should also be learned for this purpose and radiographic imaging should be avoided. In particular, with practice, the exclusion of a pneumothorax can be more reliably accomplished by sonography than by radiography.
Detailed sonography is helpful for special questions
Detailed sonography may provide additional guidance for the following questions:
Parapneumonic effusion, empyema: Parapneumonic pleural effusions develop in approximately half of all patients with pneumonia. Clinically, this can be suspected as soon as an initial pleuritic pain is suspended in pneumonia. Most of the time, they are passive, narrow and uncomplicated outpourings. However, in 10% of cases, a larger and complicated pleural effusion or pleural empyema follows. Therefore, severe pleuropneumonia in particular should be monitored by imaging during the course of treatment; ideally, an ultrasound image can then be used as a baseline finding for comparison.
Sonographically, the different stages of parapneumonic effusion and pleural empyema can be distinguished from each other well and also better than by computed tomography (Table 2).

Pleural tumors: A distinction is made between benign and malignant pleural changes. The clinically much more frequent malignant changes are divided into primary and secondary changes.
Sonographically recognizable is a widening of the parietal pleura, which can be significantly >1 cm in malignant findings, has a bumpy surface, occasionally causes nodular changes and can grow into the thoracic wall. Duplex sonography or the use of ultrasound contrast agents can detect pathologic neovascularization. The examination should be performed with a linear scanner and high sound frequency, as this provides optimum close-up distance resolution.
Pleural mesothelioma: In primary pleural malignancy, pleural mesothelioma is a rare disease. It occurs occasionally sporadically and much more frequently in association with a history of asbestos exposure. Clinical symptoms develop slowly and are initially uncharacteristic. Sonographic evidence of mesothelioma includes large tumor mass and thoracic wall infiltration (and associated pain).
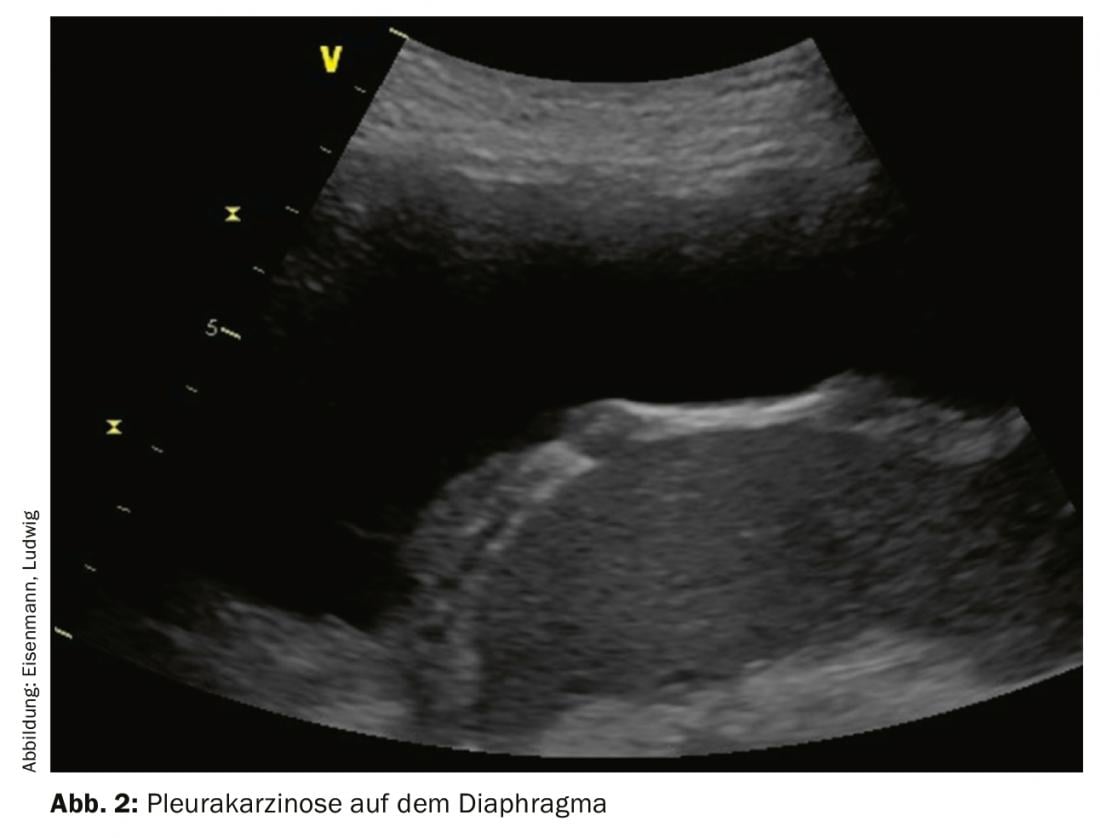
Malignant pleural effusions occur in up to 15% of all tumor patients, are associated with increased morbidity and mortality, and often mark the transition to a palliative tumor stage. Common extrathoracic causes include breast carcinoma, gastric carcinoma, and lymphoma. Malignancy can often not be detected by puncture: Sensitivity and specificity of effusion cytology are only 58% and 97%, respectively, across all tumor entities. A typical sonographic finding of the parietal pleura may help to challenge false-negative cytology findings and provide an indication for thoracoscopy. The pleura more frequently shows nodular and inhomogeneous thickening, preferably dorsobasal and also on the diaphragmatic part (Fig. 2). Widening, especially of more than 10 mm, is suspicious for malignancy until proven otherwise. In particular, unexplained large-volume and unilateral effusions should suggest malignancy.
The first step should therefore be a diagnostic effusion puncture. However, particularly in cases with a history of asbestos, alternative methods of histologic tissue collection should be sought promptly or already primarily to increase yield and safety from malignancy-susceptible pleural thickening. In case of doubt, however, thoracoscopy is indicated, in which pleurodesis can then be performed if necessary in addition to confirming the diagnosis. Prior to thoracoscopy, the positioning of the trocars should also be ensured sonographically.
Expansion capability before thoracentesis
Dyspnea is common with large-volume and rapidly recurrent pleural effusions. Before therapeutic thoracentesis, which is useful here, it must first be clarified whether the effusion and the lung can still be mobilized sufficiently, especially in the case of malignant effusion genesis. Otherwise, if the lung is tethered, there is a risk of seropneumothorax, which would consecutively refill with fluid. This constellation is called “trapped lung “. Preliminary indications may be the lack of inspiratory inflation or reduced pulse-synchronous motility, which can be documented by using M-mode. Another, albeit much more complex, option is to measure intrapleural pressure. Otherwise, if the puncture is not critical, a pneumothorax will form ex vacuo, which will rapidly fill with fluid again as a result of the lack of expansion. Also, primary chemical pleurodesis has no chance of success in this case, so that a tunneled pleural catheter should be inserted or, depending on the general condition, thoracic surgical decortication of the visceral overlying tumor masses should also be attempted.
Pneumothorax: Thoracic sonography is, especially for the exclusion of a pneumothorax, a method at least equivalent to X-ray thorax and part of the 2018 updated German Pneumothorax Guideline. The study evidence is clear, showing higher sensitivity and comparable specificity. Especially in the supine position, e.g., in patients in the intensive care unit or after interventions such as bronchoscopic tissue sampling or central venous catheterization (port, CVC), bedside ultrasound should be adopted as the method of first choice to exclude pneumothorax. The choice of transducer is usually secondary, since the characteristics listed below can be recognized with all transducer variants. Pocket devices, which can be connected to tablets or cell phones, are expected to make things even easier.
Simple features of pneumothorax
Using a schematic examination procedure, the following criteria must be worked through:
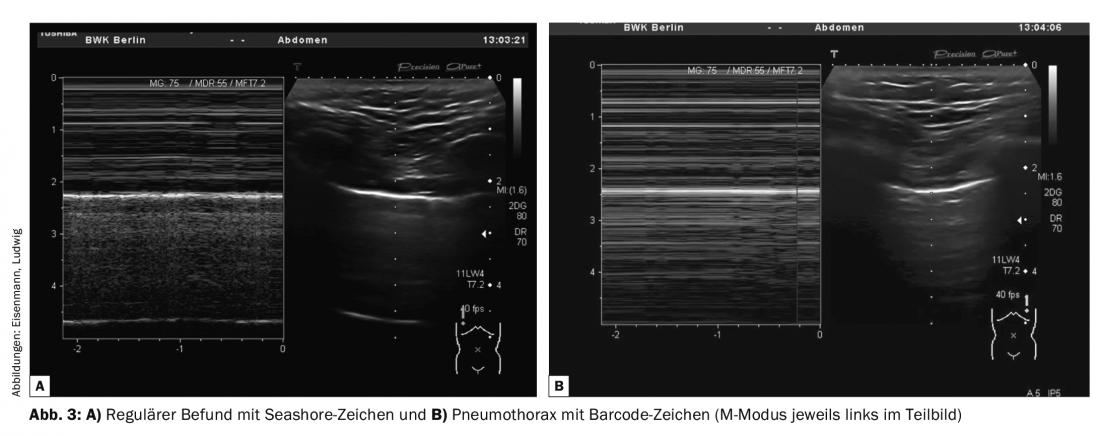
- Pleural sliding – Respiratory synchronous sliding of pleural blades, with evidence of B-lines or pleural structures if necessary. Additive use of M-mode possible with detection of Seashore sign in normal findings or barcode sign in pneumothorax (Fig. 3).
- Pulmonary pulse – Pulse synchronous pulsation of the visceral pleura, detectable directly or with the aid of color Doppler.
- Lung point – respiratory synchronously shifting transition point between pneumothorax and adjacent visceral pleura, only visible in partial pneumothorax.
The ultrasound probe is placed at the highest point of the thorax (position-dependent! In supine position: ventral in Monaldi position). If pleural sliding and/or a pulmonary pulse can be demonstrated here via the adjacent intercostal spaces, pneumothorax is virtually excluded. However, the absence of these criteria is not definite evidence of pneumothorax. In particular, pleural sliding may be severely limited or abolished in cases of pulmonary hyperinflation, large bullae, under invasive ventilation (especially jet ventilation), and pleural adhesions (after empyema or prior surgery). In the absence of evidence of pleural sliding and pulmonary pulse, a pulmonary point is then sought by lateral displacement in the respective intercostal space. If this is detectable, it proves the presence of pneumothorax.
Because of the described sources of error, the pre-interventional presentation of the initial findings is useful, and a side-by-side comparison should always be performed as well. Alternative imaging should be performed if the findings are unclear.
However, size estimation of pneumothorax is not reliably possible sonographically. The decision to treat must be made in the context of the clinical condition and genesis of the pneumothorax. Postinterventional pneumothorax, for example, can be treated conservatively if a pulmonary point is detected and the patient is asymptomatic, if clinical and sonographic monitoring can be facilitated in the short term. The location of the lung point is marked on the skin for this purpose and briefly rechecked. Thus, the dynamics of pneumothorax can be seen. The success of treatment using drainage or single-use suction can also be tracked sonographically and does not necessarily require an X-ray.
Take-Home Messages
- Sonography is the primary imaging modality used to detect and quantify pleural effusions.
- Sonographic features in the effusion, parietal pleura, or lungs are helpful for etiologic differentiation.
- Localization of the pleural effusion puncture site is required sonographically to avoid accidental injury to vascular structures and lung tissue.
- Postinterventional pneumothorax must be excluded by ultrasound at the bedside. This means that an X-ray is often unnecessary.
- A single sonography should be performed for each pneumonia. Larger parapneumonic effusions must be punctured for relief; small effusions should be monitored sonographically.
- Thoracic pain after relieved pleural puncture suggests a lack of expansile capacity in the absence of pneumothorax.
- Pneumothorax can be excluded with certainty sonographically but occasionally cannot be proven. The extent of pneumothorax cannot be assessed with certainty by sonography.
Literature:
- Beckh S, Blank W, Kubale R et al. Examination standard for transthoracic sonography Ultrasound in Medicine – European Journal of Ultrasound 2006; 27(03): 287-288.
- Volpicelli G, Elbarbary M, Blaivas M et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577-591.
- German Society of Pneumology and Respiratory Medicine (DGP), German Society of Infectiology (DGI), (PEG) P-E-GfCeV. S3 guideline “Treatment of adult patients with community-acquired pneumonia and prevention – Update 2016”. In: (AWMF) AdWMF ed. www.awmf.org/leitlinien/detail/ll/020-020.html; 2016
- Schnell J, Beer M, Eggeling S, et al: Management of Spontaneous Pneumothorax and Postinterventional Pneumothorax: German S3-Guideline. Zentralblatt fur Chirurgie 2018; 143: S12-s43.
- Joyner Jr. CR, Herman JR, Reid JM: Reflected Ultrasound in the Detection and Localization of Pleural Effusion. JAMA 1967; 200: 399-402.
- Light RW, Macgregor MI, Luchsinger PC, et al: Pleural effusions: the diagnostic separation of transudates and exudates. Annals of internal medicine 1972; 77: 507-513.
- Tasci S, Ewig S, Lüderitz B: Diagnosis and therapy of parapneumonic pleural effusions and empyema. Dtsch Arztebl 2004; 101: A 638-648.
- Bibby AC, Dorn P, Psallidas I, et al: ERS/EACTS statement on the management of malignant pleural effusions. European Respiratory Journal 2018; 52.
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al: Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. American journal of respirator y and critical care medicine 2018; 198: 839-849.
- Mathis G: Image atlas of pulmonary sonography. 6th ed. Aufl: Springer-Verlag Berlin Heidelberg; 2016.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(3): 18-22.