Current evidence suggests that the benefits of low-dose steroid therapy are greater than the harms in elderly patients with rheumatoid arthritis (RA). This is evident from the GLORIA study, in which prednisolone 5 mg/d in combination with DMARDs produced beneficial results in a placebo comparison. The use of JAK-i in elderly RA patients was evaluated in the ORAL-SURVEILLANCE study. Due to potential risks, the EMA urges caution in this patient population.
Rheumatoid arthritis (RA) is an inflammatory rheumatic systemic disease that, if left untreated, is associated with pain and progressive functional impairment. Epidemiologic data show that incidence and prevalence increase in an age-correlated manner and are highest in the age group around 70 years, explained Thomas Buttgereit, MD, Charité, University Medicine Berlin [1]. Thanks to modern treatment strategies, RA prognosis has improved, but especially in elderly patients, it is important to carefully weigh the risk-benefit profile of any given therapy [2]. Changes in pharmacokinetics and dynamics as well as the increasing number of comorbidities often make the choice of therapy a balancing act between effectiveness and risk.
Low-dose glucocorticoids: more benefit than harm
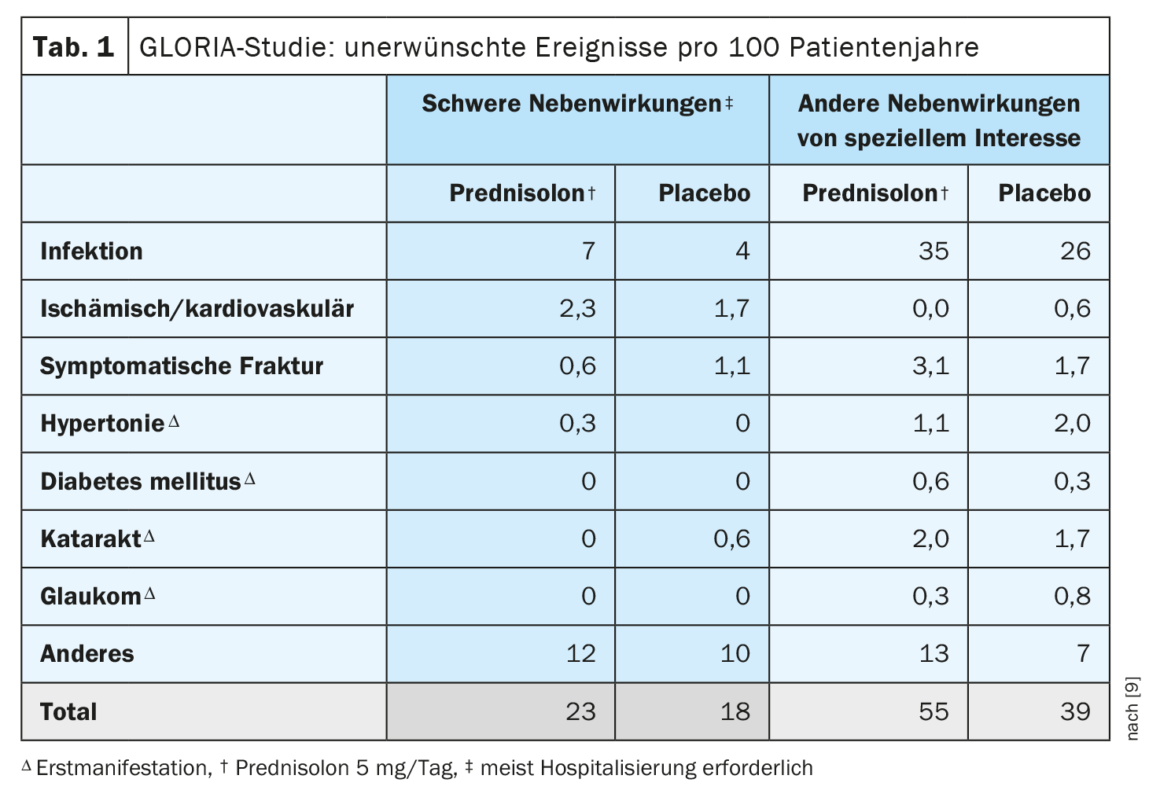
Although many modern RA treatment strategies aim to achieve glucocorticoid (GC)-free remission, GCs remain important therapeutic agents in RA, especially when administered in the short term and early in the disease course [3]. The GLORIA (Glucocorticoid LOw-dose in RheumatoId Arthritis) randomized-controlled trial published in 2022 showed no statistical difference between 5 mg prednisolone vs. placebo with respect to symptomatic fracture rates in patients over 65 years of age with established RA [4]. Further results on bone health indicate a small but significant loss of bone mineral density (BMD ) in the lumbar spine (-1%) in the prednisolone group, but no differences were observed in the hip between the prednisolone and placebo groups after an average of 19 months. However, significantly better disease activity and less progression of joint damage was demonstrated with prednisolone vs. placebo [4]. Disadvantages of prednisolone in the placebo comparison mentioned were a slightly increased fracture frequency, as well as an increase in the proportion of patients with at least one adverse event of special interest, most of which were mild to moderate infections. Overall, the results indicate that low-dose prednisolone treatment provides more benefit than harm in elderly RA patients [1]. It is important to note that the GC dose in the GLORIA study was limited to 5 mg/d prednisolone; GC at higher doses should be avoided in long-term therapy.
| The GLORIA study included ≥65-year-old* patients with RA who had at least mild arthritis activity. The objective of the study was to test the efficacy and safety of prednisolone 5 mg/d as an adjunct to conventional DMARDS. The study had two primary endpoints: first, mean disease activity over the observation period (time-averaged mean disease activity score, DAS28) and second, the occurrence of adverse events ( AEs). Secondary radiographic disease progression was analyzed. |
| * This age cutoff was chosen because seniors are at highest risk for treatment-associated harms with comorbidity and are underrepresented or even excluded from most clinical trials. |
| according to [4] |
JAK-i or biologics – caution is advised in elderly patients
ORAL-SURVEILLANCE was a randomized, open-label comparison (ratio 1:1:1) with the JAK inhibitor (JAK-i) tofacitinib at the rheumatologic dose (2× 5 mg daily) and twice the dose (2× 10 mg) and with the TNF inhibitors (TNF-i) adalimumab and etanercept [5,6]. Patients with RA aged 50 years and older who had previously had an inadequate response to methotrexate (MTX) and had at least one cardiovascular risk factor were included. A total of 4362 patients were randomized, 31% of whom were over 65 years of age. The primary endpoint of noninferiority of tofacitinib vs TNF-i with respect to serious cardiovascular events and malignancies (excluding nonmelanoma skin cancer) was missed; serious cardiac events occurred numerically more frequently with tofacitinib, as did certain malignancies (mainly pulmonary and lymphoma). Post-hoc analyses showed that predominantly certain risk groups are affected by an increased risk. These included primarily current and former smokers, age over 65 years, increased cardiac risk, or increased risk of thromboembolic events. The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) therefore recommends that patients with these characteristics should be treated with JAK-i (baricitinib, filgotinib, tofacitinib, and upadacitinib) only when no suitable alternative therapy is available to minimize the risk of serious adverse drug reactions [6,7]. If high-risk patients with JAK-i are treated, if possible, only in a reduced dosage.
The use of biologics in elderly RA patients also carries increased potential risks, as shown by a secondary analysis that included 14 studies with a total of 21985 participants, 8680 of whom belonged to the age group of ≥60 years [8]. Of these, 4719 were treated with biologics (adalimumab, infliximab, etanercept, certolizumab pegol, ustekinumab, efalizumab) , and this age group was found to have a higher risk of malignancy and mortality, as well as three times the risk of infection, compared with younger patients receiving biologics.
Congress: EULAR Annual Meeting
Literature:
- «Management of older patients with inflammatory rheumatic diseases», Dr. med. Thomas Buttgereit, EULAR Annual Meeting 31.5.–3.6.2023.
- Woolf AD, Pfleger B: Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81(9): 646–656.
- Hauser B, et al.: The Effect of Anti-rheumatic Drugs on the Skeleton. Calcif Tissue Int 2022; 111(5): 445–456.
- Boers M, et al.: For the GLORIA Trial consortium Low dose, add-on prednisolone in patients with rheumatoid arthritis aged 65+: the pragmatic randomised, double-blind placebo-controlled GLORIA trial. Ann Rheum Dis 2022;81(7): 925–936.
- Ytterberg SR, et al.: ORAL Surveillance Investigators. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med 2022; 386(4): 316–326.
- «Empfehlungen der Deutschen Gesellschaft für Rheumatologie: Januskinase-Inhibitoren (JAKi) – wie ist mit den neuen Verordnungseinschränkungen umzugehen?», Deutsche Gesellschaft für Rheumatologie, 17. März 2023.
- European Medicine Agency. Januskinase Inhibitors. www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors jaki2023, (letzter Abruf 26.06.2023)
- Borren NZ: Safety of Biologic Therapy in oder patients with Immune-Mediated Diseases: A systematic review and meta-analysis. Clin Gastroenterol Hepato 2019; 17(9): 1736–1743.
- Boers M, et al.: Favorable Balance of Benefit and Harm of Long-Term, Low Dose Prednisolone Added to Standard Treatment in Rheumatoid Arthritis Patients Aged 65+: The Pragmatic, Multicenter, Placebo-Controlled GLORIA Trial. American College of Rheumatology (ACR), 2021; Abstract Number: 1678, https://acrabstracts.org, (letzter Abruf 26.06.2023)
HAUSARZT PRAXIS 2023; 18(7): 30–32