Classes of drugs with different mechanisms of action are available for the treatment of type 2 diabetes. However, the presence of renal or cardiac insufficiency, cardiovascular disease, or insulin deficiency significantly affects therapy.
In Switzerland, about 5% of the population has diabetes, with a higher proportion in men (5.5%) than in women (3.9%) [1]. About 90% of those affected suffer from type 2 diabetes (T2D) [2]. Compared with the general population, the prevalence for this condition is particularly high in heart failure patients and is approximately 40% in individuals hospitalized with heart failure [3]. This is due in part to overlapping risk factors, pathophysiology, and comorbidities [3]. In addition, a key commonality of both diseases is that they are often associated with impaired renal function [3]. Thus, T2D and heart failure are associated with the development and worsening of chronic kidney disease, which in turn contributes to poor prognosis [3]. Because of the association between T2D, heart failure, and renal dysfunction, there is an increasing need for antihyperglycemic agents with cardio- and nephroprotective properties [3].
Available drug classes and SGED recommendations
Various classes of drugs with different mechanisms of action are available for the treatment of T2D (Table 1) . According to the recommendations of the Swiss Society of Endocrinology and Diabetology (SGED), metformin is the first-line drug of choice, with which the other available drugs can be combined early on, taking into account their mechanism of action and side effects [4]. However, several other factors, such as the presence of renal insufficiency, cardiovascular disease, heart failure, or insulin deficiency, may limit the choice of therapy [4].
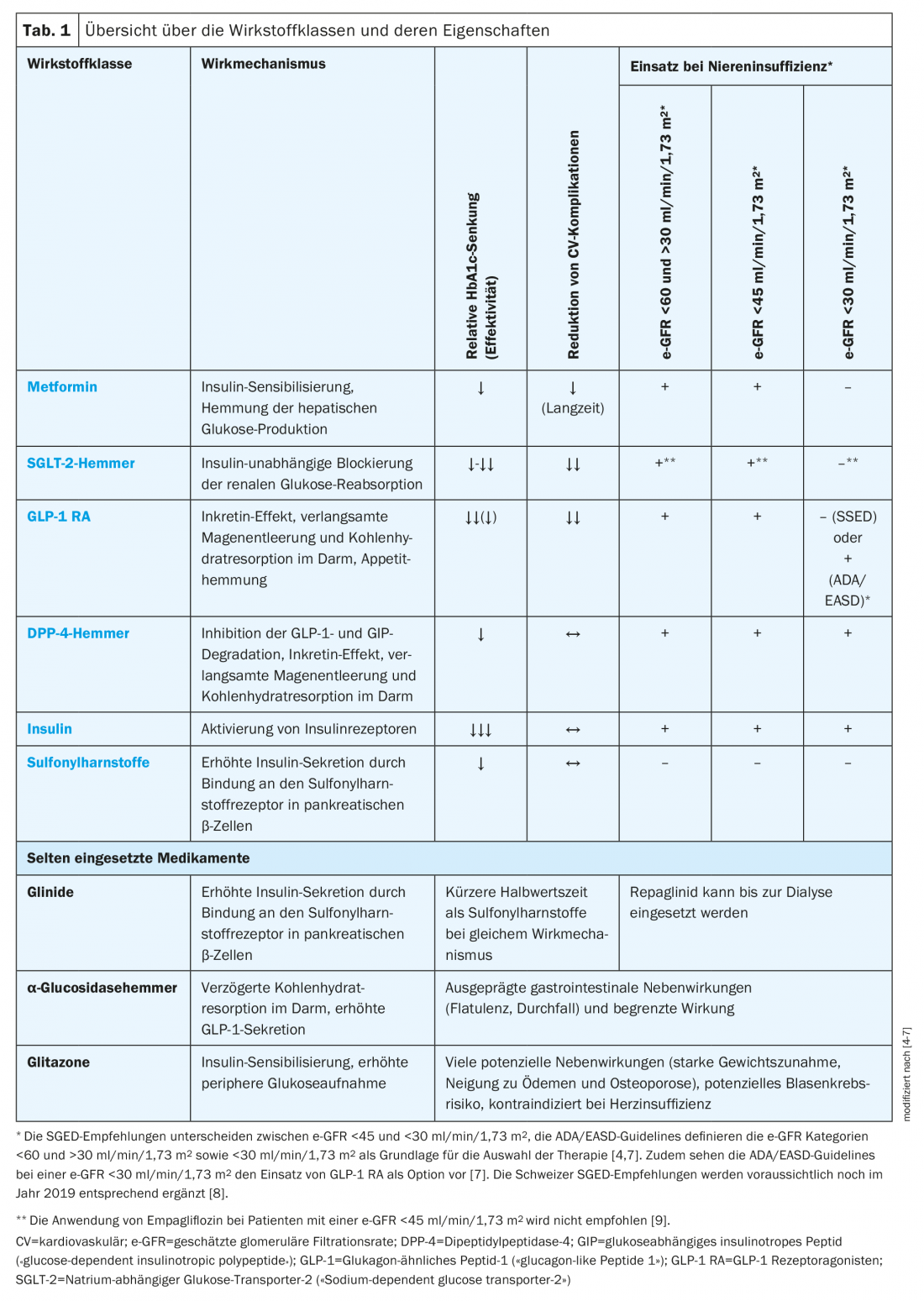
According to SGED recommendations, basal insulin is the treatment of choice for insulin deficiency, followed by mixed insulin or coformulated insulins, basal insulin in combination with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), or basal bolus therapy.
In patients with renal insufficiency, the range of drugs that can be used is also significantly limited. While long-acting sulfonylureas cannot be used in patients with moderate renal insufficiency (estimated glomerular filtration rate, e-GFR <60 but >30 ml/min/1.73m2), in severe renal insufficiency (e-GFR <30 ml/min/1.73 m2), only dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RA)1 and insulin can be considered [4,7]. In this regard, dose adjustment is required for all DPP-4 inhibitors except linagliptin, and all DDP-4 inhibitors, with the exception of saxagliptin, can be used up to dialysis. If treatment does not result in achievement of the individual HbA1c target within three months, basal insulin should be given.
With regard to cardiovascular risk, there are also differences between drug classes. Thus, metformin in long-term use and in the presence of cardiovascular disease, inhibitors of sodium-dependent glucose transporter-2 (“SGLT-2 inhibitors”) and GLP-1 RA are associated with a reduction in cardiovascular complications and even all-cause mortality [4]. Therefore, according to SGED recommendations, metformin should be used first in individuals with cardiovascular disease, with early combination with an SGLT-2 inhibitor or a GLP-1 RA indicated. If the individual HbA1c target is not achieved, the combination of metformin plus SGLT-2 inhibitor can be supplemented with a DPP-4 inhibitor, gliclazide, or basal insulin. With initial treatment with metformin plus DPP-4 inhibitors, additional medications may include gliclazide or basal insulin.
In individuals without cardiovascular disease, treatment with metformin in combination with an SGLT-2 inhibitor, GLP-1 RA, or DPP-4 inhibitor is possible. The latter can be supplemented with gliclazide or basal insulin if needed.
If heart failure is present, treatment with metformin plus SGLT-2 inhibitor is indicated initially. If necessary, an additional DPP-4 inhibitor can be used in the further course. If this does not lead to the desired treatment success, basal insulin should be used (Fig. 1) [4].
Focus heart
SGLT-2 inhibitors: in addition to their primary antihyperglycemic effect, SGLT-2 inhibitors possess several properties that may favorably influence cardiovascular prognosis [10]. Thus, positive effects on cardiovascular events have been demonstrated in various cardiovascular outcome studies in patients with T2D. In the EMPA-REG OUTCOME study of patients with established cardiovascular disease, empagliflozin was superior to placebo treatment for the primary composite cardiovascular endpoint (pooled data, Tab. 2). In addition, empagliflozin reduced cardiovascular mortality, all-cause mortality (HR 0.68; 95% CI 0.57-0.82; p<0.001), and the risk of hospitalization for heart failure (HR 0.65; 95% CI 0.50-0.85; p=0.002) [10,11]. In the composite endpoint of hospitalization or death due to heart failure, empagliflozin also showed a positive effect (HR 0.61; 95% CI 0.47-0.79; p<0.001) [12]. Based on these data, empagliflozin is indicated by Swissmedic “for the prevention of cardiovascular events in patients with type 2 diabetes mellitus and already manifest cardiovascular disease” [9].
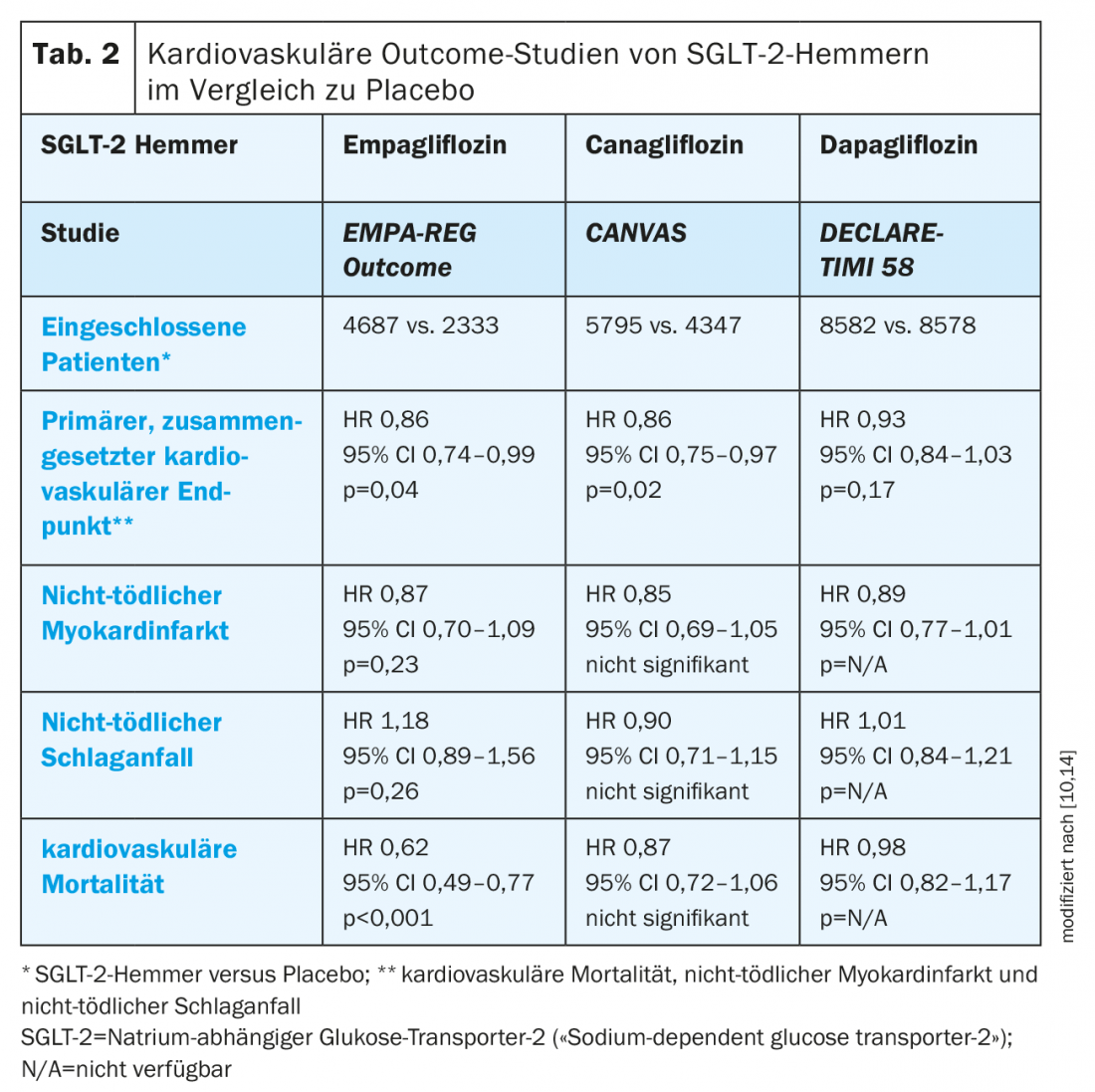
Canagliflozin also showed a reduction in the primary composite cardiovascular endpoint in patients with cardiovascular disease or associated risk factors in the CANVAS trial program, but failed to achieve significance with respect to cardiovascular mortality [10,13]. In addition, an increased risk of amputations was observed [13].
Recently presented data from the DECLARE-TIMI 58 study showed a statistically significant reduction in hospitalizations due to heart failure in patients with cardiovascular disease or associated risk factors taking dapagliflozin (2.5% vs. 3.3% on placebo; p<0.005), and in the composite endpoint of hospitalization due to heart failure or cardiovascular death (4.9% vs. 5.8% with placebo, p=0.005). In addition, dapagliflozin achieved noninferiority but not superiority to placebo with respect to major cardiovascular events. In contrast to canagliflozin, no increased risk of amputation was observed with dapagliflozin [14]. In addition, it is important to note that patients in the DECLARE-TIMI 58 trial had significantly better e-GFR values compared with the EMPA-REG Outcome and CANVAS trials because of different exclusion criteria, which contributed to the lower mortality in the DECLARE-TIMI 58 trial [11,13,14].
GLP-1 RA: The ELIXA trial demonstrated noninferiority of GLP-1 RA lixisenatide versus placebo with respect to the primary composite cardiovascular end point but no beneficial effect on cardiovascular outcome (Table 3) [15].
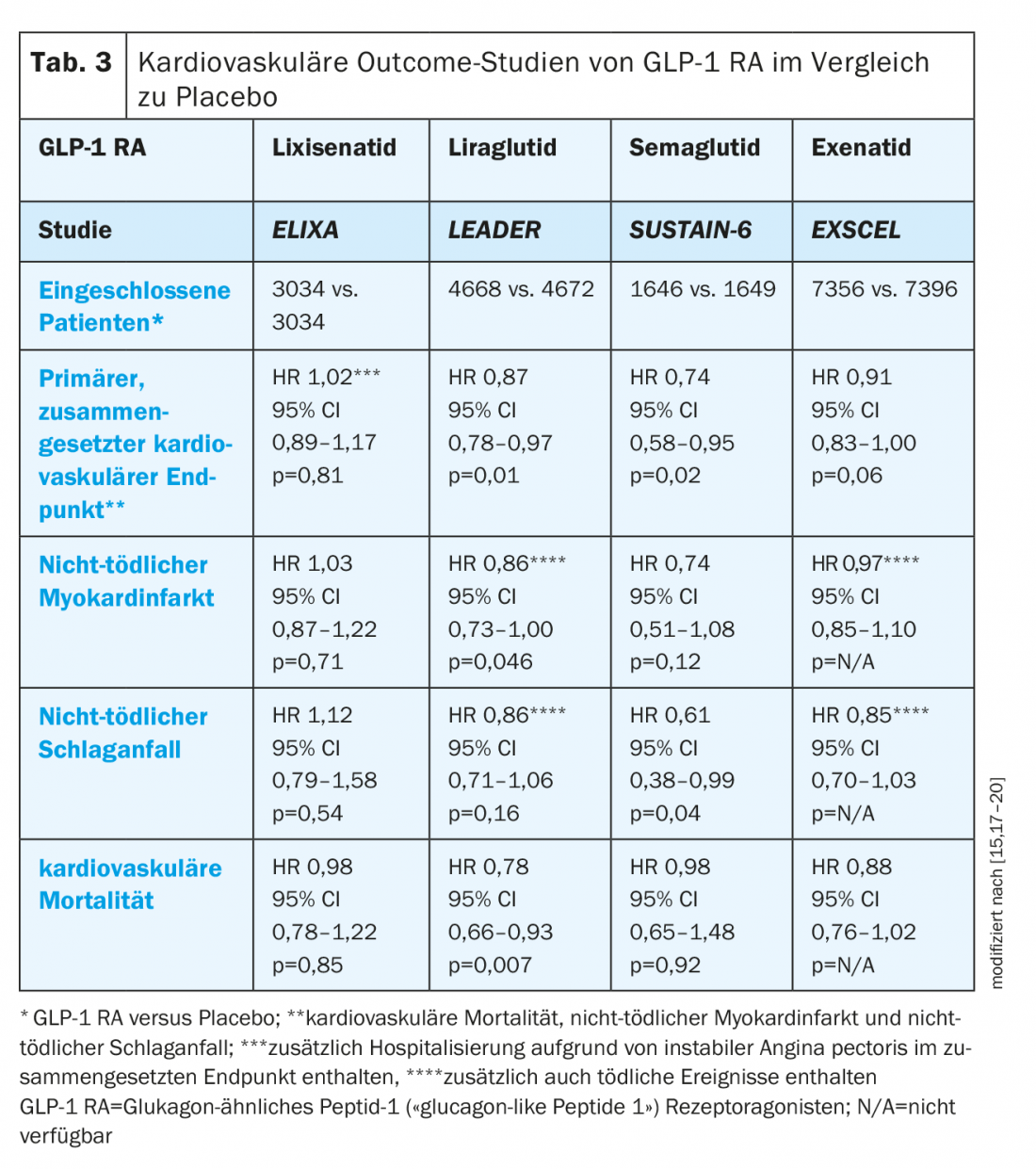
In contrast, the LEADER trial demonstrated both noninferiority of liraglutide to placebo and a significant reduction in cardiovascular mortality in patients with cardiovascular disease or associated risk factors. All-cause mortality was also significantly reduced, whereas there was no difference in hospitalization for heart failure [15]. Based on these data, liraglutide is indicated by Swissmedic for the “prevention of cardiovascular events in patients with type 2 diabetes mellitus and already manifest cardiovascular disease” [16].
The noninferiority of semaglutide to placebo was confirmed in the 3-point MACE, showing possible superiority (3-point MACE: 26% reduction; p<0.001 for noninferiority; p=0.02 for superiority), although the study was not designed to evaluate superiority. In addition, a significant reduction in nonfatal stroke and myocardial infarction, but no effect on cardiovascular or all-cause mortality was observed [17].
Once-weekly treatment with exenatide was noninferior to placebo in the EXSCEL trial but failed to show differences in cardiovascular mortality, incidence of nonfatal myocardial infarction or stroke, and hospitalization for heart failure [15,17–20].
DPP-4 inhibitors: positive cardiac and vascular effects of DPP-4 inhibitors were observed in preliminary studies, and initial results from phase II and phase III trials suggested a reduction in cardiovascular events by these agents [10]. Cardiovascular outcome studies have demonstrated non-inferiority of alogliptin, saxagliptin, sitagliptin, and linagliptin to placebo (Table 4) . It should be noted that the patients included in these trials were already receiving standard of care regarding T2D management and cardiovascular risk management [10].
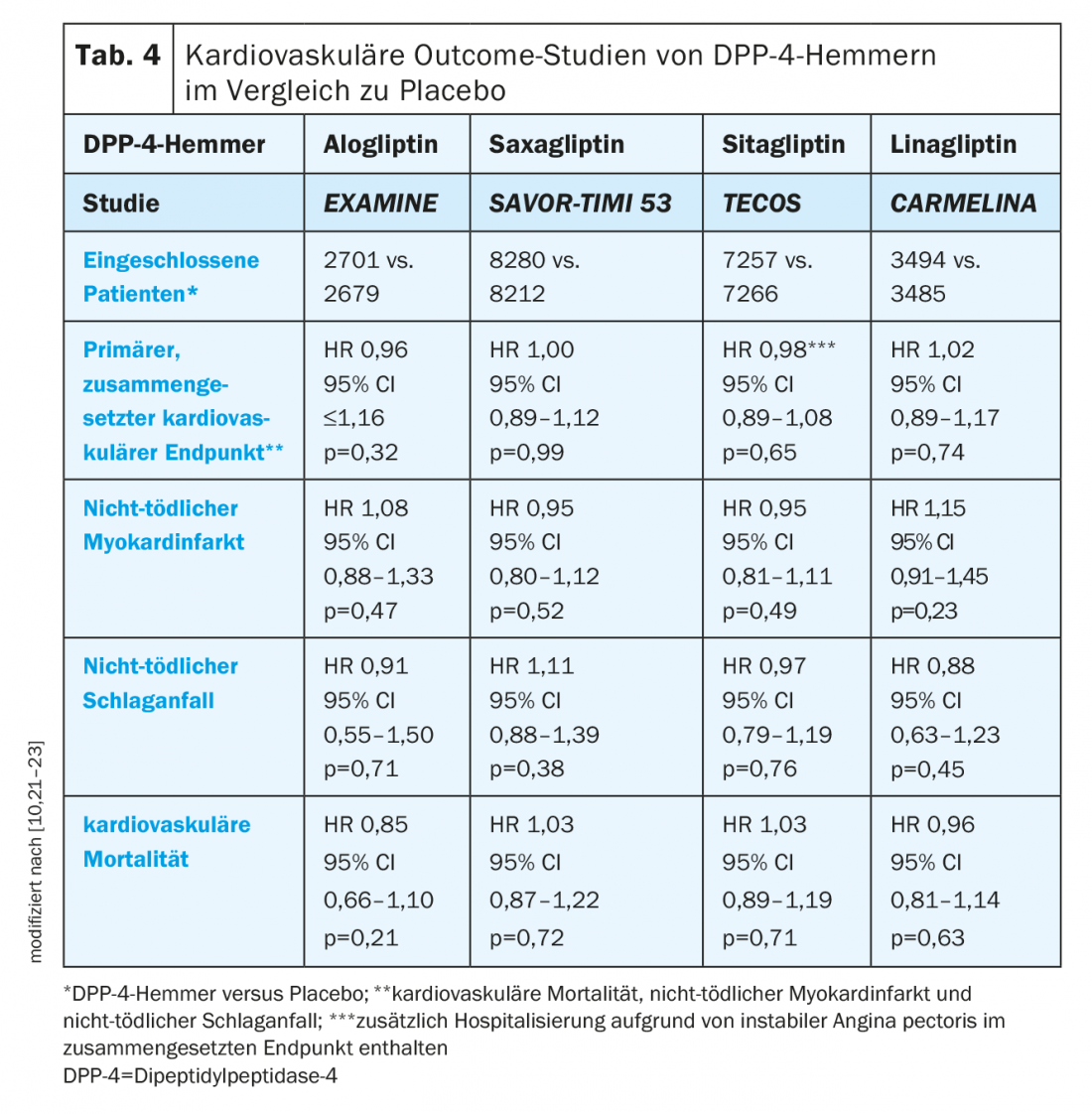
An open question regarding the use of DPP-4 inhibitors is their impact on the occurrence of heart failure. In the EXAMINE trial, there was a non-significant trend for a higher hospitalization rate due to heart failure with alogliptin (1.07; 95% CI 0.79-1.46; p=0.657) [10,24]. However, a post-hoc analysis found no effect on the composite endpoint of cardiovascular death and hospitalization for heart failure (HR 1.00; 95% CI 0.82-1.21) [24].
In the SAVOR-TIMI 53 trial, significantly more patients in the saxagliptin group were hospitalized with heart failure than on placebo (3.5% vs. 2.8%; HR 1.27; 95% CI 1.07-1.51; p=0.007) [22]. Among these, heart failure risk was highest in T2D patients with elevated levels of natriuretic peptide, prior heart failure, or chronic kidney disease at baseline [25].
In contrast to two previous studies, hospitalization rates did not differ between the sitagliptin and placebo groups in the TECOS study (HR 1.00; 95% CI 0.83-1.20; p=0.98) [10,26].
Linagliptin also did not result in an increased hospitalization rate due to heart failure in the CARMELINA study (HR 0.90; 95% CI 0.74-1.08; p=0.2635) [21].
Focus kidney
SGLT-2 inhibitors: empagliflozin as well as canagliflozin and dapagliflozin showed potential nephroprotective properties in the respective outcome studies, although the patient populations differed in each study (Table 5) [3]. Whereas the EMPA-REG Outcome and CANVAS studies included patients with an e-GFR of at least 30 ml/min/1.73 m², the DECLARE-TIMI 58 study included only patients with an e-GFR >60 ml/min/1.73 m² [11,13,14].
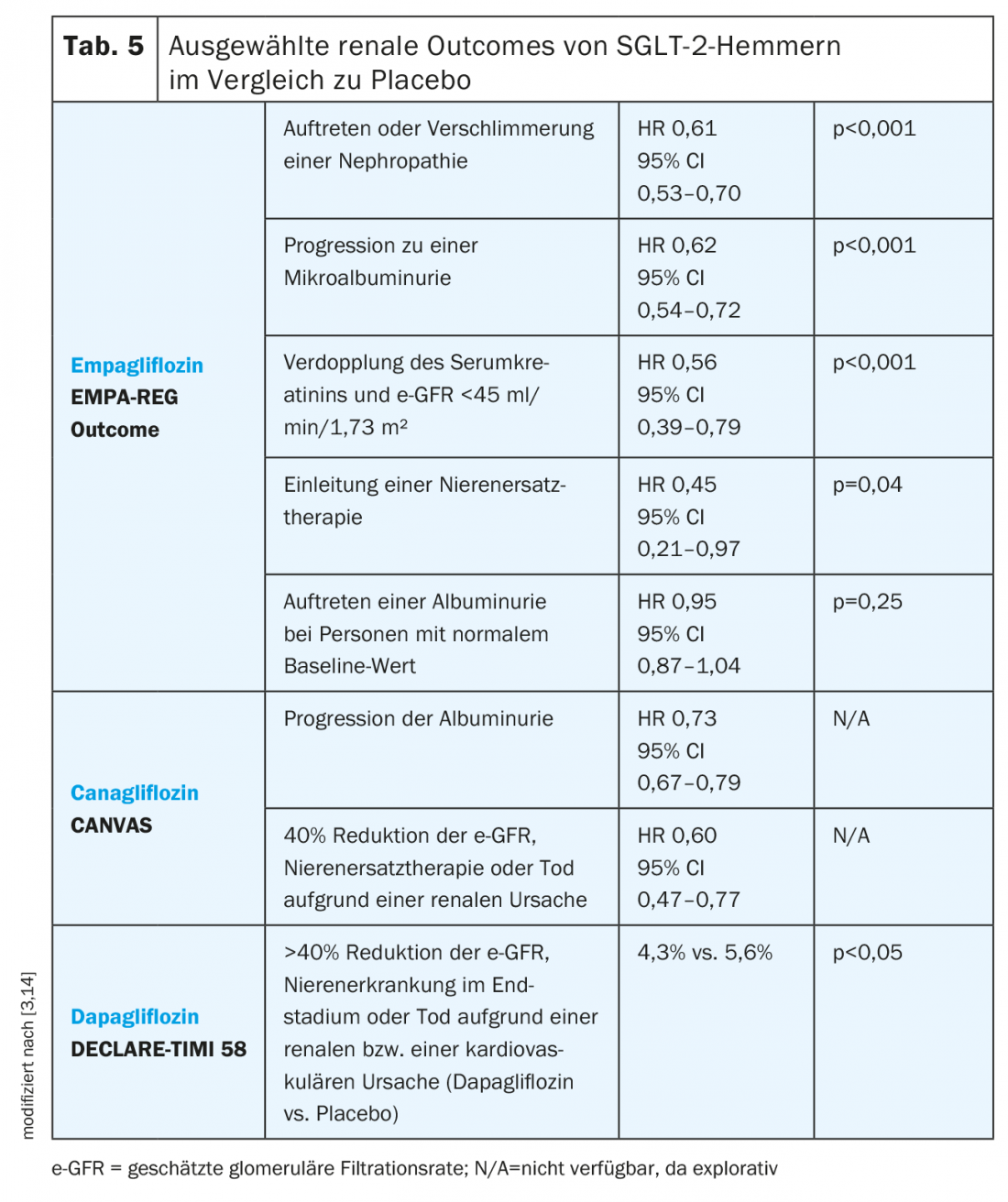
In the EMPA-REG Outcome Study, empagliflozin was associated with a lower incidence of worsening nephropathy, which was defined as progression to microalbuminuria, doubling of serum creatinine with an e-GFR <45 ml/min/1.73 m², initiation of renal replacement therapy, or death from renal disease. All individual components, except death from kidney disease, reached statistical significance. In the empagliflozin group, e-GFR decreased moderately during the first four weeks but then stabilized and returned to baseline at the end of therapy, whereas e-GFR in the placebo group decreased continuously throughout the study [3].
In the CANVAS study, progression of albuminuria, defined as a greater than 30% increase in albuminuria and a change from normo- to microalbuminuria or micro- to macroalbuminuria, was lower in the canagliflozin group than with placebo. In addition, there was a reduction in the composite endpoint of 40% reduction in e-GFR, renal replacement therapy, or death from a renal cause with canagliflozin compared with placebo [3]. Renal outcomes of canagliflozin are also currently being investigated in the CREDENCE study [27].
As recently presented data from the DECLARE-TIMI 58 trial show, dapagliflozin also has a beneficial effect on renal outcomes and is associated with a reduction in the composite endpoint >40% reduction in e-GFR, end-stage renal disease, or death from a renal or a cardiovascular cause [14].
GLP-1 RA: In the ELIXA study, there was no significant effect of GLP-1 RA lixisenatide on the urinary albumin-creatinine ratio after accounting for HbA1c (Table 6) [3].
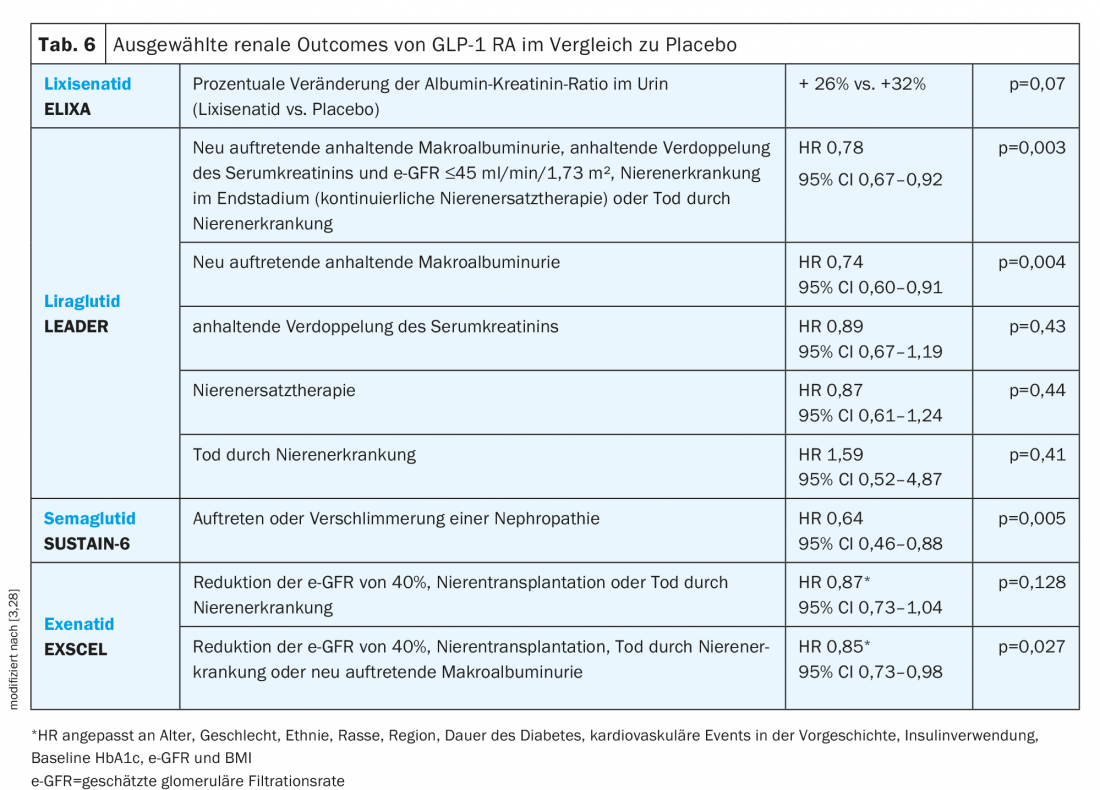
In contrast, liraglutide treatment in the LEADER trial resulted in a significantly lower risk for the composite renal endpoint. Moreover, despite an overall reduction, e-GFR was moderately better in the liraglutide group than placebo at 36 months (e-GFR ratio 1.02; 95% CI 1.00-1.03) [3].
In the SUSTAIN-6 trial, patients on semaglutide were shown to have a lower risk of nephropathy, which was defined as macroalbuminuria, sustained doubling of serum creatinine, and an e-GFR <45 ml/min/1.73 m² or continuous renal replacement therapy [3].
Recently presented data from the EXSCEL study showed a significant reduction in the composite renal endpoint consisting of reduction in e-GFR of 40%, renal transplantation, death from renal disease, or new-onset macroalbuminuria with exenatide in an adjusted analysis. Macroalbuminuria occurred in 2.2% of patients in the exenatide group and in 2.5% in the placebo group. No difference in e-GFR was observed (“least squares mean difference” +0.21; 95% CI -0.27 to 0.70 ml/min/1.73 m², p=0.39) [28].
DPP-4 inhibitor: In the TECOS trial, treatment with the DPP-4 inhibitor sitagliptin was associated with a slight but sustained decrease in urinary albumin-creatinine ratio throughout the 3-year follow-up (Table 7) [29]. Decreased albuminuria was also observed with saxagliptin in the SAVOR-TIMI 53 study [3]. With regard to e-GFR, a slightly but consistently lower value was observed in the TECOS study under sitagliptin than under placebo. This was not the case in the SAVOR-TIMI 53 or EXAMINE studies [24]. Here, this sustained reduction differs from the initial decrease and subsequent stabilization of e-GFR with empagliflozin observed in the EMPA-REG outcome study [3].
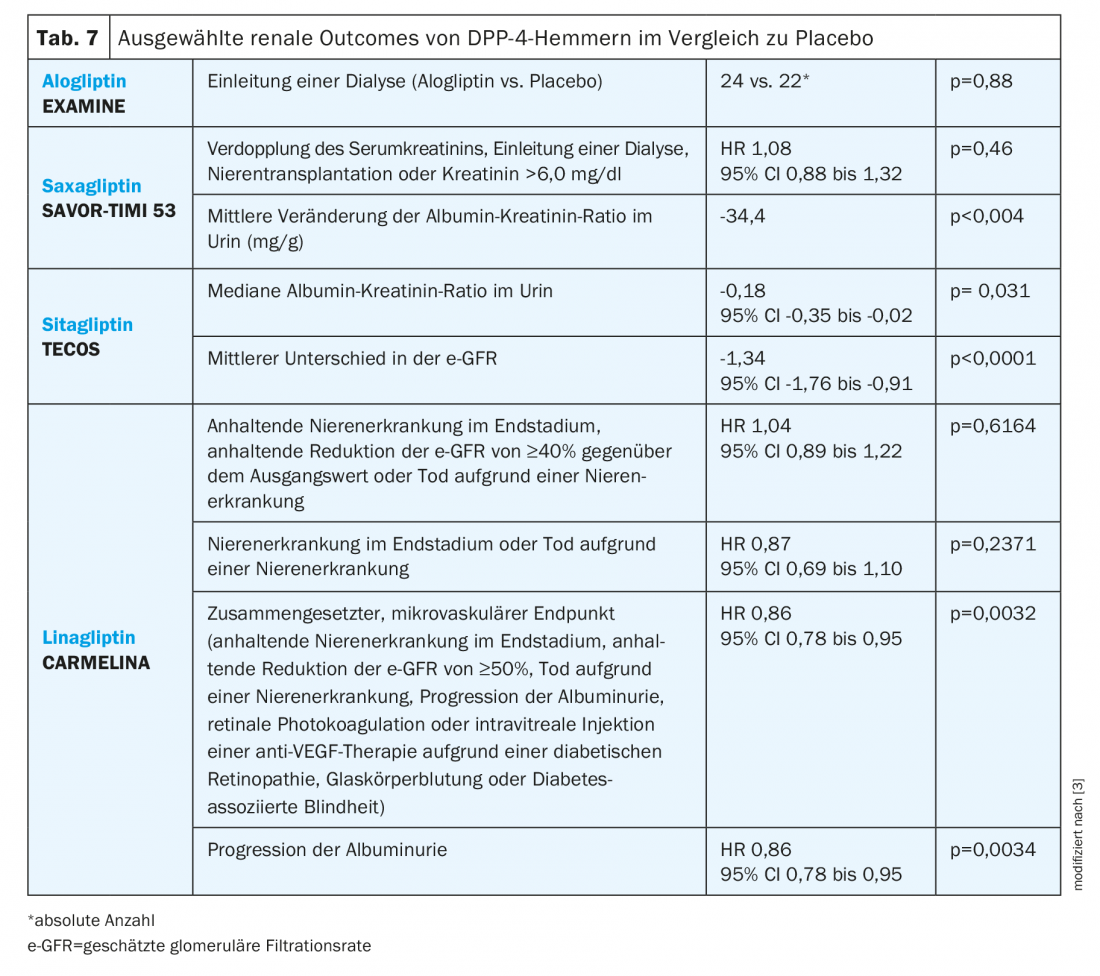
The CARMELINA study evaluated patients with cardiovascular disease and/or chronic kidney disease, confirming both cardiovascular and renal safety. In addition, there were significant differences compared with placebo in the composite microvascular end point and with respect to progression of albuminuria [21].
Selection of antidiabetic therapy
Based on cardiovascular safety data and potential protective properties, SGED recommendations suggest that SGLT-2 inhibitors or the GLP-1 RA liraglutide, semaglutide, or dulaglutide should be preferred in the presence of cardiovascular disease. Sulfonylureas should not be used in patients with moderate renal insufficiency (e-GFR <45 ml/min/1.73 m2) [4]. In severe renal insufficiency (e-GFR<30 ml/min/1.73 m2), the choice of antidiabetic agents is limited to insulin, GLP-1 RA1, and DPP-4 inhibitors, although the use of DPP-4 inhibitors requires dose adjustment for almost all agents in this group except linagliptin (Table 8) [4,7].
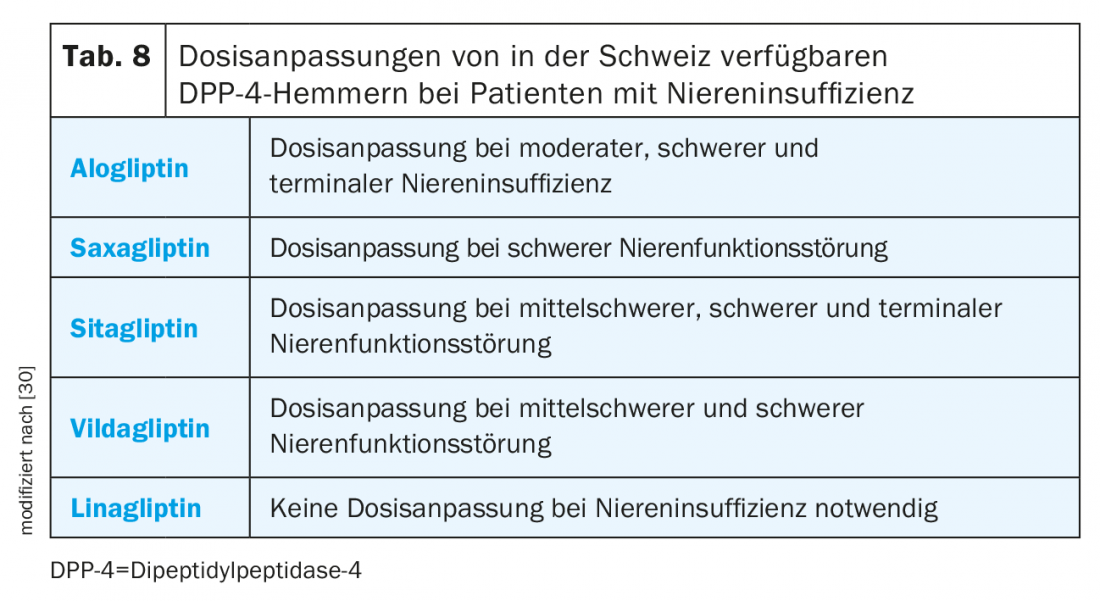
SGLT-2 inhibitors should not be used with an e-GFR <30 ml/min/1.73 m2 because as renal function declines, so does the blood glucose-lowering effect. However, the beneficial effects on preserving renal function and reducing cardiovascular events and mortality are maintained up to an e-GFR of 30 ml/min/1.73m2 [4].
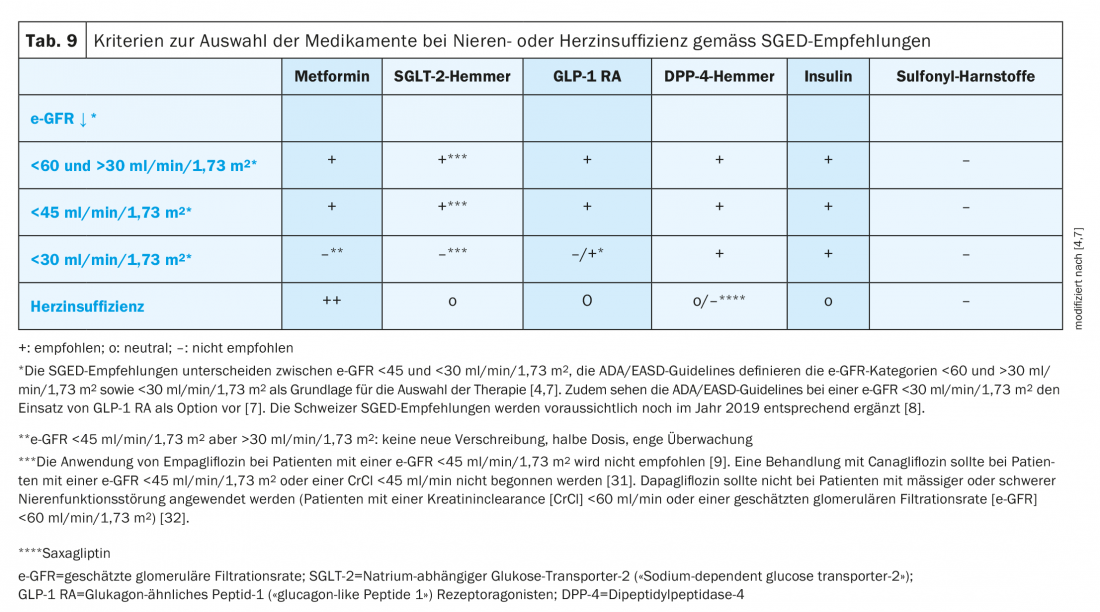
An overview of the criteria for therapy selection in renal or cardiac failure according to SGED recommendations is shown in Table 9 [4].
Patients with cardiac and renal insufficiency
Many epidemiological observations have been able to show an association between cardiovascular morbidity and mortality and decreased renal function. This is irrespective of which of the two conditions occurs first, with the term cardio-renal or reno-cardiac syndrome being used depending on the initial trigger. Both pathologies show some overlap in this regard and may each be favored by diabetes [33]. The classification of this pathology distinguishes five different types, with the first four types describing acute or chronic cardio-renal or reno-cardiac syndromes. Type 5, on the other hand, refers to secondary cardio-renal syndromes or cardio-renal involvement in systemic diseases. Accordingly, one can speak of a type 5 cardio-renal syndrome in the field of diabetes [34].
Regardless of classification, the question in clinical practice is which drug class should be used in T2D patients with concomitant heart and renal failure. If the severity of renal insufficiency permits, SGLT-2 inhibitors are initially the treatment of choice. If renal function continues to decline, GLP1-RA can then be considered. In severe renal failure, only DDP-4 inhibitors, GLP1-RA1, and insulin are indicated. Overall, it should be noted that diuretics must be used carefully in these patients to avoid overdiuresis [4,7].
Take-Home Messages
- Classes of drugs with different mechanisms of action are available for the treatment of type 2 diabetes: Metformin, SGLT-2 inhibitors, GLP-1 RA, DPP-4 inhibitors, insulin, and sulfonylureas.
- According to SGED recommendations, metformin is the first-line drug of choice, with which the other drugs can be combined early on.
- The presence of renal insufficiency, cardiovascular disease, heart failure, or insulin deficiency can significantly influence the choice of therapy.
- In patients with cardiovascular disease, SGED recommendations state that an SGLT-2 inhibitor (empagliflozin, dapagliflozin, canagliflozin) or a GLP-1 RA (liraglutide, semaglutide, dulaglutide) should be preferred.
- In severe renal insufficiency (e-GFR<30 ml/min/1.73m2), the choice of antidiabetic agents is limited to insulin, GLP-1 RA1, and DPP-4 inhibitors, with all DPP-4 inhibitors except linagliptin requiring dose adjustment.
Literature:
- Swiss Federal Statistical Office: Diabetes. www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/krankheiten/diabetes.html, last accessed 29 Oct. 2018.
- Diabetes Switzerland: Diabetes mellitus. www.diabetesschweiz.ch/diabetes/, last accessed 10/29/2018.
- McHugh, KR, et al: The emerging role of novel antihyperglycemic agents in the treatment of heart failure and diabetes: A focus on cardiorenal outcomes. Clin Cardiol 2018; 41(9): 1259-1267.
- Lehmann, R, et al: Recommendations of the SGED/SSED: Measures for blood glucose control in patients with type 2 diabetes mellitus. Swiss Society of Endocrinology and Diabetology 2016.
- Chaudhury A, et al: Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Frontiers in endocrinology 2017; 8: 6. 10.3389/fendo.2017.00006
- Stein SA, Lamos EM, Davis, SN: A review of the efficacy and safety of oral antidiabetic drugs. Expert opinion on drug safety 2013; 12(2): 153-175.
- Davies MJ, et al: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018; 61(12): 2461-2498.
- Lehmann R: Personal communication from Prof. R. Lehmann- Chairman of the SGED/SSED Working Group.
- Swissmedic: Current product information Jardiance®. www.swissmedicinfo.ch. Status of the information: April 2018.
- Scheen AJ: Cardiovascular Effects of New Oral Glucose-Lowering Agents: DPP-4 and SGLT-2 Inhibitors. Circulation Research 2018; 122(10): 1439-1459.
- Zinman B, et al: Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine 2015; 373(22): 2117-2128.
- Fitchett D, et al: Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. European Heart Journal 2016; 37(19): 1526-1534.
- Neal B, et al: Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine 2017; 377(7): 644-657.
- Wiviott SD, et al: Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. England J. Med 2019; 380: 347-357. doi: 10.1056/NEJMoa1812389. Epub 2018 Nov
- Cefalu WT, et al: Cardiovascular Outcomes Trials in Type 2 Diabetes: Where Do We Go From Here? Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care 2018; 41(1): 14-31.
- Swissmedic: Current expert information Victoza®. www.swissmedicinfo.ch, Date of information: April 2018.
- Marso SP, et al: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2016; 375(19): 1834-1844.
- Holman RR, et al: Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine, 2017; 377(13): 1228-1239.
- Marso SP, et al: Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2016; 375(4): 311-322.
- Pfeffer MA, et al: Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. New England Journal of Medicine 2015; 373(23): 2247-2257.
- Rosenstock J, et al: Cardiovascular and Renal Microvascular Outcome Study with Linagliptin in Patients with Type 2 Diabetes Mellitus (CARMELINA®). Oral presentation at the 54th Annual Meeting of the European Association for the Study of Diabetes (EASD), Berlin, Thursday, October 4, 2018. https://www.easd.org/virtualmeeting/home.html#!contentsessions/2873.
- Scirica BM, et al: Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. New England Journal of Medicine 2013; 369(14): 1317-1326.
- White WB, et al: Alogliptin after acute coronary syndrome in patients with type 2 diabetes. New England Journal of Medicine 2013; 369(14): 1327-1335.
- Zannad F, et al: Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. The Lancet 2015; 385(9982): 2067-2076.
- Scirica BM, et al: Heart failure, saxagliptin and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014; 130(18): 1579-1588.
- Green JB, et al: Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2015; 373(3): 232-242.
- Jardine MJ, et al: The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. American journal of nephrology 2017; 46(6): 462-472.
- Bethel MA, et al: Renal outcomes in the EXenatide Study of Cardiovascular Event Lowering (EXSCEL). Diabetes, 2018. 67(Supplement 1): https://doi.org/10.2337/db18-522-P
- Cornel JH, et al: Effect of Sitagliptin on Kidney Function and Respective Cardiovascular Outcomes in Type 2 Diabetes: Outcomes From TECOS. Diabetes Care 2016; 39(12): 2304-2310.
- Swissmedic: www.swissmedicinfo.ch.
- Swissmedic: Current product information Invokana®. www.swissmedicinfo.ch, as of April 2018.
- Swissmedic: Current expert information Forxiga®. www.swissmedicinfo.ch, Date of information: January 2018.
- Schrier RW: Cardiorenal versus renocardiac syndrome: is there a difference? Nat Clin Pract Nephrol 2007; 3(12): 637.
- Di Lullo L, et al: Type-5 cardiorenal syndrome (CRS-5): an up to date. Nephrology@ Point of Care 2017; 3(1): p. e23-e32, https://doi.org/10.5301/napoc.5000212
HAUSARZT PRAXIS 2019; 14(3): 18-27
CARDIOVASC 2019; 18(2): 16-23











