Hypertrophic cardiomyopathy (HCM) is the most common hereditary heart muscle disease. The clinical picture ranges from asymptomatic patients to manifest heart failure and sudden cardiac death (SCD). The mainstay of management is risk stratification for SCD and indication for primary prophylactic ICD implantation.
Hypertrophic cardiomyopathy (HCM) is the most common hereditary heart muscle disease. The diagnosis is made when there is asymmetric thickening of the myocardium with a wall thickness of at least 15 mm and after exclusion of other cardiac or extracardiac causes [1]. The clinical presentation ranges from completely asymptomatic patients to symptoms such as chest pain, dyspnea, palpitations, dizziness, and syncope to manifest heart failure and sudden cardiac death. Pathophysiologically, there is a complex interplay between diastolic dysfunction, microcirculatory dysfunction, and left ventricular outflow tract (LVOT) obstruction. LVOT obstruction is common, which can usually be successfully treated with drug therapies but occasionally requires invasive therapies such as septal alcohol ablation or surgical myectomy [2]. Systolic function is usually preserved to hyperdynamic, but may also decline during the “burn-out” phase; approximately 5-10% of patients develop manifest systolic dysfunction during the course [3].
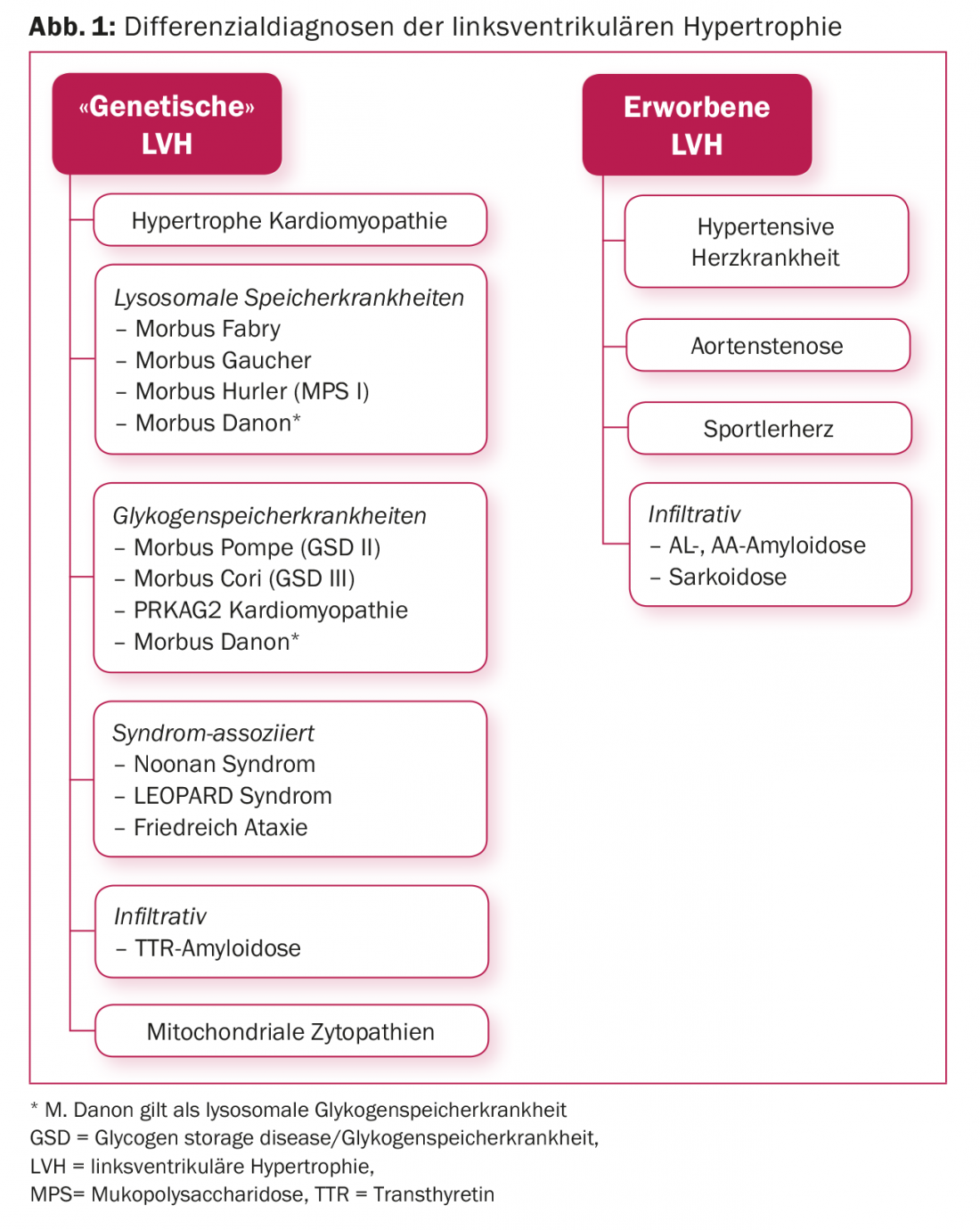
Primarily, the problem of correct diagnosis arises when imaging (echocardiography and/or cardiac MRI) reveals left ventricular hypertrophy. (Fig.1). The diagnostic process is a so-called “multi-modality approach” and should take into account information from family history (inheritance), personal history, clinical examination (evidence of systemic disease), ECG (pre-excitation), laboratory, echocardiography, cardiac MRI, etc. Correct diagnosis is important as this has implications for the further management of the patient as well as their relatives. If the diagnosis of HCM cannot be confirmed after exhausting conventional diagnostic means, genetic analysis may be helpful, the importance of which lies on the one hand in the confirmation/exclusion of so-called “phenocopies” and on the other hand in enabling family screening should a pathogenic mutation be found. If hypertrophic cardiomyopathy can be confirmed after all investigations, risk stratification for sudden cardiac death is one of the most important pillars for the further management of patients.
General genetic aspects
The prevalence of HCM is 1:500 and the mode of inheritance is autosomal dominant. To date, far >1400 mutations have been identified whose pathogenicity for HCM genesis is regarded as certain and whose localization is mostly in the sarcomeric protein genes (Tab. 1). It is important to distinguish benign alterations or variants of unclear significance from pathogenic mutations. Close collaboration between clinicians and cardiogeneticists is of utmost importance, as clinicians depend on correct classification of mutations. This is an ever-evolving field, and in addition to establishing familial segregation, it is always necessary to check the mutations found against international databases, as mutations can be reclassified. In case of unclear mutations, it is therefore of utmost importance to continue regular clinical family screening with ECG and echocardiography.

Genetic testing in HCM is not a mandatory benefit of health insurance and the costs are only covered if a therapeutic consequence for the affected patient can be demonstrated. An application for approval of the costs must therefore be submitted to the health insurance company in advance. Since 2017, panel examinations can only be ordered by physicians with FMH Genetics to ensure expertise in the interpretation of results, which in the case of HCM should be a cardiogeneticist.
A disease-causing mutation is found in approximately 40-60% of index patients tested. Of these, >80% are located in either the beta-myosin heavy chain (MYH7) or myosin-binding protein C (MBPC3) gene. In most cases, a mutation occurs with replacement of one normally functioning amino acid by another. However, there are also more radical alterations such as insertions or deletions of nucleotides [4] and in rare cases two pathogenic mutations are present [5]. Patients with two pathogenic mutations have been described to have more severe disease courses with more pronounced hypertrophy and more frequent need for heart transplantation. Overall, however, no stringent genotype-phenotype correlation has yet been established and single mutations cannot be used for risk stratification [6].
The same genetic mutation can lead to varying degrees of disease manifestation within a family. The spectrum ranges from negative phenotype to marked left ventricular hypertrophy and sudden cardiac death (SCD). The causes for this are unclear; environmental influences and other modifying factors such as epigenetic factors are discussed.
Whether genetic testing is useful in a patient with left ventricular hypertrophy must be decided on an individual basis, taking into account clinical presentation, family history, and knowledge of what genetics may be useful for. The diagnostic hit rate of genetic testing can be estimated based on morphology, extent of hypertrophy, family history, and presence/absence of arterial hypertension (“Toronto HCM genotype score”) [7].
Family screening for HCM
First-degree family members have a 50% risk of inheriting at least the genetic predisposition to develop HCM. Therefore, clinical family screening also plays a central role in counseling. This includes an ECG and echocardiography every 3-5 years, and every 12-18 months during adolescence, as the disease can manifest at any age, but is most clustered during length growth in adolescence.
Performing genetics is recommended especially in large families with a clinically clear picture, as a pathogenic mutation facilitates family screening. In the case of a confirmed HCM-causing mutation, genetic screening of first-degree relatives may be performed. It is important that clinical screening with ECG and echocardiography is also performed at about the same time. On the one hand, to assess a clinical manifestation of the disease, and on the other hand, of course, to establish familial segregation, which ultimately also represents a certain quality control with regard to the pathogenicity of the mutation. Family members who are clinically and genetically negative may, according to the current state of knowledge, be discharged from the follow-up examinations; always provided, of course, that the familial mutation is clearly pathogenic.
Finally, it should be mentioned that in reproductive medicine, in vitro fertilization now offers the possibility of pre-implantation diagnostics, which gives couples the opportunity to have a child who is not a carrier of the genetic defect.
HCM Phenocopies
In many cases, one is initially dealing with an unclear left ventricular hypertrophy. Differentiating hypertrophic cardiomyopathy from other conditions that may mimic HCM (= phenocopies) is important because each condition shows an individual clinical course, requires specific therapy, and is often associated with a prognostically less favorable course compared with HCM. In addition to a detailed medical history, it is important to analyze imaging and laboratory parameters in detail, as these can be indicative (Fig. 2) . If left ventricular hypertrophy remains unclear after a comprehensive clinical evaluation, genetic testing is recommended, since all HCM panels today include the genes of so-called phenocopies (Table 2).

Genotype-positive/phenotype-negative constellation
Previously, it was assumed that any patient carrying a pathogenic mutation would also develop left ventricular hypertrophy. Today it is known that the penetrance of HCM is not 100%, although ultimately no exact figures are yet available in the literature. Thus, there are always cases that have a positive genotype but negative phenotype. These should be clinically examined annually to avoid missing any disease manifestation. These patients may pass on the genetic predisposition, which is why their children should also be examined clinically and also genetically. Patients with positive genotype and negative phenotype are the subject of current debates in expert circles [8] and are handled differently in international guidelines. In North America, competitive sports are allowed under close monitoring [9]. In Europe, on the other hand, competitive sports are discouraged, but with regard to recreational sports, restrictions are cautious [10]. It is also assumed that there is an increased tendency to cardiac arrhythmias, but this has not been proven and there are no recommendations for primary prophylactic ICD implantation.
Sudden Cardiac Death (SCD) Risk Stratification.
The incidence of SCD is 0.6% per year in HCM patients compared to 0.3% per year in the normal population [11]. HCM is the most common cause of sudden cardiac death in young athletes, accounting for one-third of deaths [12]. It is up to the treating clinician to identify patients who are at increased risk for sudden cardiac death and treat them accordingly. In secondary prevention of SCD, discussions regarding the indication for implantation of a cardioverter defibrillator (ICD) rarely occur. In the primary prophylaxis setting, patients must be evaluated regularly and the indication for an ICD must be considered individually. European and U.S. HCM experts have disagreed on the risk stratification of SCD for several years, resulting in separate treatment guidelines (Fig. 3).

Based on the individually determined criteria, according to the European Guidelines, a 5-year risk of sudden cardiac death can be calculated using the HCM risk calculator to guide the decision regarding ICD therapy [1]. Young patients with marked hypertrophy, predescribed nonsustained ventricular tachycardia, unexplained syncope, positive family history, large left atrium, and LVOT obstruction are at high risk.
The American guidelines divide risk factors into major factors and modifying and minor factors [13]. The main factors are considered to be sudden cardiac death of a first-degree family member, marked hypertrophy, and unexplained syncope in the previous six months. If any of these criteria are met, there is a class IIa indication for ICD implantation. In the absence of major factors, at least two minor factors must be present to recommend primary prophylactic ICD implantation. There are always patients who do not fall into a clear category, in this case modifying factors can be used to support decision making (Fig. 3) . The main differences between the European and American treatment guidelines are that the Europeans use continuous numbers, whereas the Americans classify the factors as categorical. In addition, it should certainly be considered that in severe LVOT obstruction, the therapy of choice is septal reduction therapy rather than implantation of an ICD. Evaluation of patients in a center of excellence for HCM is recommended, especially in unclear cases.
If the indication for ICD implantation arises, the choice of device must be made carefully and tailored to the individual patient. Storage disease or infiltrative cardiomyopathies are at risk of higher-grade AV block, so a transvenous system with pacing and defibrillation modes should be implanted. In such cases, a subcutaneous ICD is not an option. If, on the other hand, impaired ejection fraction with dyssynchrony is already present, resynchronization therapy may be considered.
To keep the risk of malignant arrhythmias low, sports restrictions are imposed on patients with manifest HCM. The consensus is that competitive level sports activities are not recommended. Likewise, intense physical activities associated with high sympathetic tone should be avoided (so-called “high-sprint/high-burst activities” such as soccer, ice hockey, tennis, etc.).
Summary
The great advances in genetic research in recent years now allow us to better understand hypertrophic cardiomyopathy and to target genetic testing in patient management. These are particularly helpful in differential diagnostic considerations as well as family screening. However, in the current era, the latter often presents us with the dilemma of the gene type-positive/phenotype-negative patient, with insufficient current data to answer elementary questions: how at risk is the gene carrier without clinically manifest left ventricular hypertrophy? How drastic should the sporting restrictions be? Thus, the most important pillar in the care of HCM patients remains the risk stratification of SCD, which is based on a detailed history and examination including multi-modality imaging. The fact that the American and European guidelines do not always provide uniform recommendations makes one realize that there is a large gray area that requires the experience of a specialist and in which each therapeutic decision must be individually adapted to the patient.
Take-Home Messages
- HCM is the most common heritable myocardial disease and is diagnosed when the wall thickness exceeds 15 mm, as long as other diseases that may lead to left ventricular hypertrophy have been excluded.
- A comprehensive evaluation and diagnosis includes a detailed (family) history, ECG, laboratory, HOLTER examination, ergometry, echocardiography, and cardiac MRI when possible.
- Genetic screening is helpful in confirming/excluding the diagnosis or differentiating it from so-called phenocopies and is also highly valued in family screening.
- Genotype-positive/phenotype-negative patients are a separate group and data regarding their management is limited.
- The most important pillar in management is risk assessment for sudden cardiac death and indication for primary prophylactic ICD implantation.
Literature:
- Elliott PM, et al: 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35(39): 2733-2792.
- Maron MS, et al: Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006; 114: 2232-2239.
- Olivotto I, et al: Patterns of Disease Progression in Hypertrophic Cardiomyopathy. An Individualized Approach to Clinical Staging. Circulation: Heart Failure 2012; 5: 535-546.
- Ho CY, et al: Genetic advances in sarcomeric cardiomyopathies: state of the art. Cardiovasc Res 2015; 105(4): 397-408.
- Fourey D, et al: Prevalence and Clinical Implication of Double Mutations in Hypertrophic Cardiomyopathy: Revisiting the Gene-Dose Effect. Circulation: Genomic and Precision Medicine 2017; 10:e001685.
- Pasquale F, et al: Long-term outcomes in hypertrophic cardiomyopathy caused by mutations in the cardiac troponin T gene. Circ Cardiovasc Genet 2012; 5(1): 10-17.
- Gruner C, et al: Toronto hypertrophic cardiomyopathy genotype score for prediction of a positive genotype in hypertrophic cardiomyopathy. Circ Cardiovasc Genet 2013; 6(1): 19-26.
- Maron BJ, Yeates L, Semsarian C: Clinical challenges of genotype positive (+)-phenotype negative (-) family members in hypertrophic cardiomyopathy. Am J Cardiol 2011; 107(4): 604-608.
- Maron BJ, Zipes DP, Kovacs RJ: Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Preamble, Principles, and General Considerations: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015; 66(21): 2343-2349.
- Pelliccia A, et al: Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005; 26(14): 1422-1445.
- Maron MS, et al: Contemporary Natural History and Management of Nonobstructive Hypertrophic Cardiomyopathy. J Am Coll Cardiol 2016; 67(12): 1399-1409.
- Maron BJ, et al: Sudden death in young athletes. Circulation 1980; 62(2): 218-229.
- Gersh BJ, et al: 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124(24): 2761-2796.
CARDIOVASC 2018; 17(1): 14-20