Ginkgo-containing preparations can alleviate neuropsychiatric complaints and are widely used, especially for the treatment of cognitive performance deficits. But what about the combinability with anticoagulant agents? In a recently published drug-drug interaction study in healthy volunteers, a special phytopharmacological extract of Ginkgo biloba had no effect on either the pharmacokinetics or the pharmacodynamics of the antithrombotic agent rivaroxaban.
According to the European Medicines Agency (EMA), ginkgo extracts can be used for the treatment of cognitive performance impairment as well as for the alleviation of arterial circulatory disorders, vertigo, and tinnitus [1]. EGb 761® is a special Ginkgo biloba extract recommended by guidelines for the treatment of mild to moderate dementia. In addition, “mild cognitive impairment” (MCI) and vertigo are among the evidence-based indications [2–4]. In terms of combinability with other drug interventions, investigation of the potential for interaction is of interest. A recent study addressed the question of whether a single or repeated administration of 240 mg EGb 761® affects the pharmacokinetics or dynamics of rivaroxaban in healthy volunteers [5]. Rivaroxaban is an active substance from the group of direct oral anticoagulants (DOAK). It is a direct factor Xa inhibitor used for the prophylaxis and treatment of thromboembolic diseases.
|
In the present study, neither bleeding events nor any hemorrhage-related adverse events were observed [5]. No changes in relevant laboratory parameters (erythrocyte count, hemoglobin, hematocrit, platelet count, urinary hemoglobin, or urinary erythrocytes) were manifested that would indicate blood loss following the use of rivaroxaban in combination with EGb 761®. Reported adverse events were predominantly mild or moderate, with headache being the most common. |
Plasma level curves did not differ significantly
The monocentric, two-part study was designed as follows: In part 1 (days 1-7), rivaroxaban was administered as monotherapy [5]. In Part 2 (Days 8-15), subjects received rivaroxaban on the first and last day of each eight-day treatment with EGb 761®. Plasma levels of rivaroxaban and anti-factor Xa activity were measured up to 48 h after each intake of the anticoagulant agent. A total of 41 healthy subjects (25 male, 16 female) aged 21-70 years were analyzed for data. The plasma concentration-time curve was used to evaluate pharmacokinetics because changes in plasma concentration may be indicative of drug-drug interactions. The analyses revealed a high concordance of pharmacokinetic profiles with rivaroxaban administration alone compared with parallel treatment with EGb 761® for both single and repeated administration of the Ginkgo special extract (day 8 and day 15, respectively) (Table 1).
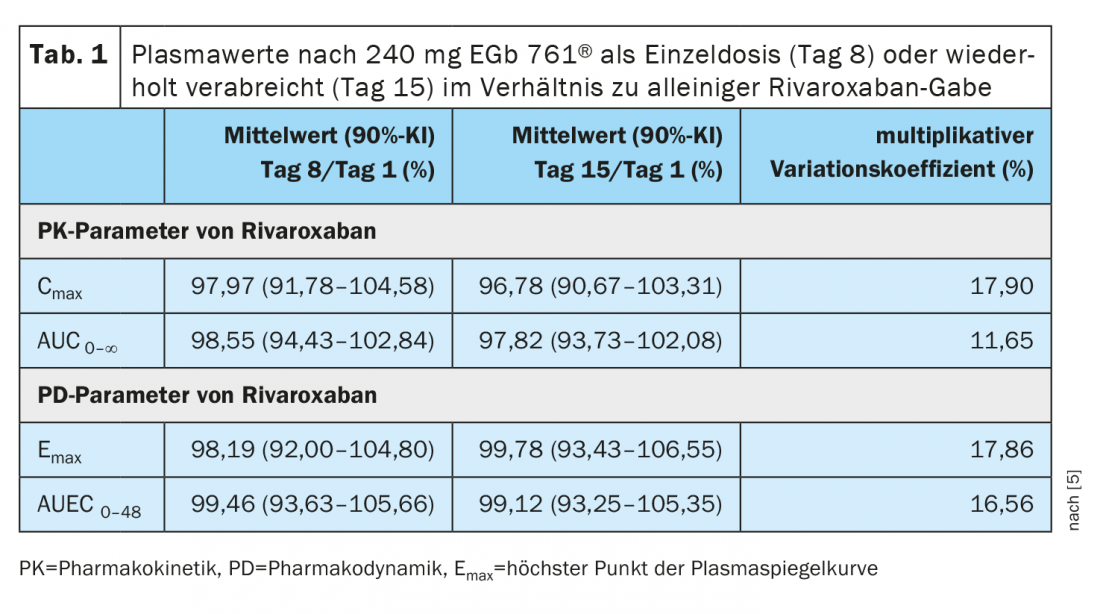
There were no significant differences from Cmax* and AUC0-∞ # in plasma on day 1, day 8, and day 15 (Table 1).Emax (highest point of the plasma level curve) and AUEC0-48 (t0 to 48 h after drug administration) also differed little on days 1, 8, and 15, with estimated means ranging from 96.78 to 99.46. It can be concluded that the intake of EGb 761® did not have a relevant effect onEmax or AUEC0-48 either as a single dose or when administered repeatedly. of rivaroxaban. Furthermore, a clear overlap of the pharmacodynamic profiles of rivaroxaban was manifested on days 1, 8, and 15 as indicated by the course of anti-factor Xa activity of rivaroxaban measured by chromogenic assay. The results of the present study are consistent with findings of Mueck et al. and Kreutz et al. in which parallel use of EGb 761® and rivaroxaban also had no effect on the pharmacokinetics of rivaroxaban [6,7].
* Cmax = highest plasma concentration after drug administration.
# AUC (“Area Under the Curve”) = Area under the curve
Literature:
- EMA, www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-ginkgo-biloba-l-folium_en.pdf (last accessed May 02, 2022).
- Drug Compendium, https://compendium.ch, (last accessed May 02, 2022).
- DGPPN/DGN: S3-Leitlinie “Demenzen”, 2016, long version. www.dgppn.de (last call 02.05.2022)
- Băjenaru O, et al.: Effectiveness and Safety Profile of Ginkgo Biloba Standardized Extract (EGb 761®) in Patients with Amnestic Mild Cognitive Impairment. CNS Neurol Disord Drug Targets. 2021, Feb 8, (online ahead of print), doi: 10.2174/1871527320666210208125524.
- Hoerr R: Single and Repeated Doses of EGb 761® do not Affect Pharmacokinetics or Pharmacodynamics of Rivaroxaban in Healthy Subjects. Front Pharmacol, 20 April 2022, https://doi.org/10.3389/fphar.2022.868843
- Mueck W, et al: Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban. Clin Pharmacokinet 2013; 53(1): 1-16.
- Kreutz R, et al: Dissociation between the Pharmacokinetics and Pharmacodynamics of Once-Daily Rivaroxaban and Twice-Daily Apixaban: a Randomized Crossover Study. J Thromb Haemost 2017; 15(10): 2017-2028.
HAUSARZT PRAXIS 2022; 17(5): 37











