For the treatment of type 2 diabetes mellitus, incretin mimetics have been used successfully for many years. GLP-1 receptor agonists, which mimic the intestinal hormone glucagon-like peptide 1 (GLP-1), not only lower blood glucose, but also suppress appetite and slow gastric emptying. This mechanism of action promotes sustained weight loss, as shown by various clinical studies. Liraglutide is already on the market for this indication in Switzerland and many other countries, and regulatory submissions for semaglutide are currently under review.
The drug Saxenda® (liraglutide) has been approved in Switzerland since 2020 for the treatment of obesity in patients with a BMI ≥ 30 kg/m² or ≥ 27 kg/m² if concomitant diseases such as prediabetes, diabetes mellitus type 2, arterial hypertension or dyslipidemia are present [1]. Another substance that has demonstrated great potential for the indication area of weight loss is semaglutide – also a GLP-1 receptor agonist (GLP-1-RA). At the annual meeting of the European Association for the Study of Diabetes (EASD), experts discussed recent study findings in the context of obesity therapy and type 2 diabetes [2]. The focus was on phase III data from the STEP program, which confirmed the efficacy of semaglutide for durable weight loss (box) [3–5]. Semaglutide is a long-acting GLP-1 RA that, unlike liraglutide, only needs to be administered once weekly [6].
|
Phase III study program “STEP In the STEP program, approximately 5000 study participants were randomized to semaglutide up to 2.4 mg per week or placebo [3,4]. In the STEP-1 and STEP-2 studies, the mean change in body weight from baseline to week 68 was -14.9% in the semaglutide group and -2.4% [4,5] in the placebo group. Thus, significantly higher weight loss was achieved under semaglutide. Overall, high effect sizes were achieved with semaglutide 2.4 mg, far exceeding previous pharmacological obesity therapies. In the dosage of 1 mg per week, semaglutide (s.c.) is already established in the treatment of type 2 diabetes, and an application for an extension of marketing authorization for semaglutide (s.c.) 2.4 mg has been submitted to the European Medicines Agency (EMA) for the indication of obesity. |
Tricking the yo-yo effect with weight loss drugs
Dr. Priya Sumithran, University of Melbourne (AUS), explained that although lifestyle interventions are an important measure for overweight individuals, lifestyle modification alone often does not lead to sustainable weight loss in the longer term, but instead results in a yoyo effect [2]. Bariatric surgery (e.g. gastric bypass) is an effective way to lose weight permanently on a larger scale. However, the hurdles are great, firstly because this surgery is only available for those with a BMI >35 kg/m2 and secondly because very few patients want such an invasive procedure. Medications are a welcome alternative to achieve a reduction in body weight and thus an overall improvement in health by pharmacotherapeutic means. Ideally, these are combined with lifestyle measures in the area of nutrition and exercise.
Semaglutide drops pounds and improves HbA1c levels
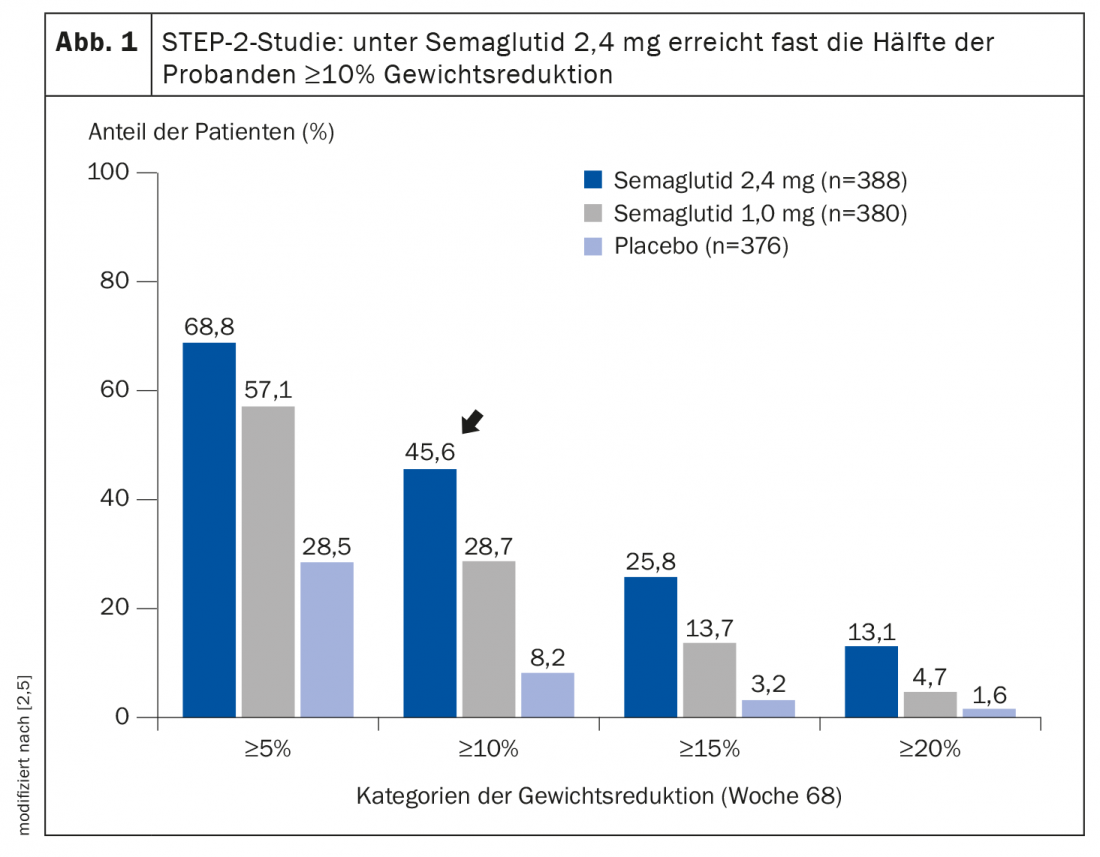
In the double-blind STEP-2 trial published in the Lancet, semaglutide in subcutaneous form was compared with placebo, in each case in combination with a lifestyle program [5]. Adults with a body mass index (BMI) of at least 27 kg/m2 and an HbA1c level of 7-10% (53-86 mmol/mol) who had been diagnosed with type 2 diabetes at least 180 days before screening were included. The mean age of study participants at baseline was 55 years, the mean body weight was 99.8 kg, and the mean duration of diabetes was 8.0 years. After the 68-week study period, 68.8% of patients treated with semaglutide 2.4 mg (n=388) achieved at least 5% weight loss, compared with only 28.5% in the placebo group (n=376). This difference proved to be highly statistically significant. Most remarkably, however, a proportion of 45.6% in the semaglutide group achieved at least 10% weight reduction (Fig. 1), which correlated with excellent HbA1c levels. Dr. Sumithran highlighted the relevance of this finding, saying, “In type 2 diabetes, remission is an achievable goal for many patients, especially if they achieve sustained weight loss of 10% or more.” With semaglutide 2.4 mg, compared with placebo and with semaglutide 1.0 mg, more patients achieved both HbA1c<7.0% and weight loss ≥10%, as well as reduced use of antihyperglycemic medications [7]. The weight loss achieved with semaglutide 2.4 mg was accompanied by significant improvements in insulin resistance and β-cell function, two key mechanisms in the pathophysiology of type 2 diabetes [7].
Congress: EASD Annual Meeting 30.9.2021
Literature:
- Swissmedicinfo: www.swissmedicinfo.ch, last accessed 08.12.2021
- Sumithran P: Harnessing weight loss interventions for treatment of type 2 diabetes. Dr. Priya Sumithran, EASD-Lancet symposium press conference, 09/30/2021.
- Blüher M: Juggling calories alone often falls short in obesity. Info Diabetol 2021; 15(5): 34-41.
- Wilding JPH, et al: STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021; 384(11): 989.
- Davies M, et al: STEP 2 Study Group Semaglutide 2-4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021; 397(10278): 971-984.
- Lau J, et al: Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem 2015; 58: 7370-7380.
- Pedersen SD, et al: Effect of semaglutide 2.4 mg on glucose metabolism and body weight in adults with overweight or obesity and type 2 diabetes in the STEP 2 trial. OP 04 GLP-1 receptor agonism: higher dose, combination therapy, or both? 57 th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia 2021, 64, 1-380.
HAUSARZT PRAXIS 2022; 17(1): 18-19