Surgery is the first choice for localized gastric carcinoma. Systems therapy peri- or postoperatively can improve cure rates. Palliative, it prolongs survival and improves quality of life.
The statements in this article apply to both classic gastric carcinoma and gastroesophageal transition (GEJ) tumors because these two entities have generally been studied together in recent trials.
Curative intended system therapy
The cure rate for stage 1 gastric cancer is approximately 70% with R0 resection. It already drops to a good 35% in stage 2 disease with surgery alone. To increase cure rates, the use of preoperative and/or postoperative systemic therapies has been evaluated in clinical trials. For curative intentional systemic therapy, there is the American model (postoperative [adjuvante] radiochemotherapy) [1] and the European model (perioperative [neoadjuvante und adjuvante] chemotherapy) [2].
The American INT0116 trial treated patients after resection of gastric cancer with five weeks of radiochemotherapy with 5FU and two doses of 5FU at monthly intervals [1]. Median survival was 36 months in the adjuvant radiochemotherapy group and 27 months with surgical therapy alone (although the study has been criticized for inadequate surgical staging of lymph node stations and consequent potential overestimation of treatment benefit). Notwithstanding the debate about the adequate extent of lymph node resection, adjuvant radiochemotherapy analogous to the INT0116 trial continues to be widely used in the Americas-particularly when interdisciplinary discussion occurs only after surgery. As an alternative to adjuvant radiochemotherapy, the ARTIST and CLASSIC trials have shown that chemotherapy alone with cisplatin/capecitabine resp. Oxaliplatin/capecitabine established [3,4]. Both studies have certain drawbacks, so that the strategies pursued in them are not widely applied in Europe. The ARTIST trial compared chemotherapy alone with radiochemotherapy (rather than observation alone), and the CLASSIC trial was conducted in exclusively Asian patients.
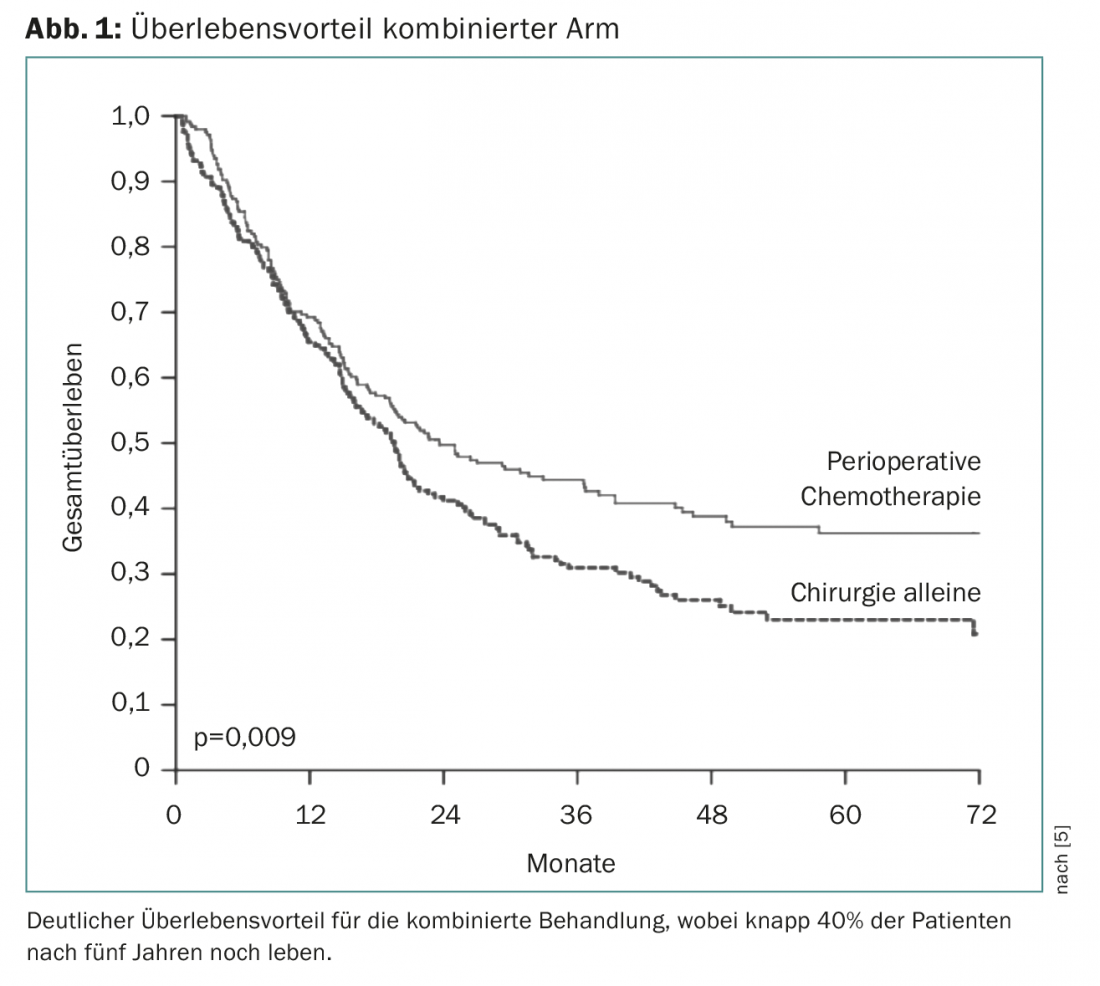
Perioperative chemotherapy first became established with the European MAGIC trial. This investigated chemotherapy with three cycles of epirubicin, cisplatin, and 5FU (ECF) before surgery and three cycles of ECF after surgery vs. surgery alone [5]. This showed a clear survival advantage for the combined arm, so that this treatment became the standard of care from 2006 (Fig. 1) . Perioperative therapy has been further confirmed as the principle (vs. chemotherapy alone) in the FNLCC/FFCD trial or the EORTC-40954 trial. These studies differ in the duration of perioperative chemotherapy as well as in the substance composition. The type of perioperative chemotherapy was advanced primarily in the AIO-FLOT4 trial, which compared three effective agents (5FU, oxaliplatin, docetaxel) over four cycles before and after surgery against the previous Magic trial standard (ECF/ECX) before and after surgery. This showed a clear survival benefit from 35 to 50 months with surprisingly good tolerability of perioperative FLOT chemotherapy, making this regimen an unchallenged standard to date [6].
The American model historically arose from the fact that patients were not presented to the interdisciplinary team until after surgery. Today, cases are usually discussed preoperatively at the interdisciplinary tumor board. Based on the available data, perioperative systemic therapy should now be considered starting at stage cT2cN0. The following reasons speak for this approach:
- The stage is not infrequently more advanced than determined by staging studies
- Prognosis is usually determined by distant metastasis and much less frequently by local recurrence
- Postoperative chemotherapies can sometimes not be applied at all or only partially (delayed postoperative recovery).
- The main studies that tested perioperative chemotherapies included patients with stage cT2cN0 and above.
Currently, a large phase III trial (FLOT5 trial) is investigating whether patients with gastric/GEJ tumor and limited metastases should benefit from surgery (perioperative chemotherapy) or be treated by FLOT chemotherapy alone [7]. Considering the improved surgical techniques and the very promising FLOT4 precursor study [8], the question of this study is very interesting. Unfortunately, the first study results are not expected until 2022.
Palliative system therapy
Balancing treatment benefits against treatment-associated side effects is, as always, critical in the palliative setting. Palliative systemic therapy leads in principle to a prolongation of survival and an improvement in quality of life. There is no clear standard for first-line treatment. Usually, a two-drug combination (e.g., 5FU/oxaliplatin) or, if the patient is in good general condition and highly motivated for therapy, a three-drug combination such as the FLOT therapy mentioned above is used. In the studies, therapy was generally administered without pause until progression, severe side effects, or discontinuation of therapy by the patient. In practice, the therapy is very rarely carried out for longer than twelve months, but usually only for six months.
Approximately 20-25% of gastric cancers are HER2 positive. Following the TOGA trial, these patients still receive standard first-line therapy with cisplatin, 5FU (or capecitabine), and trastuzumab [9]. The degree of HER2 amplification has some predictive value for treatment response and survival. Trastuzumab prolongs survival by nearly three months compared with cisplatin and 5FU/capecitabine plus placebo. Unfortunately, unlike breast carcinoma, newer HER2-targeted therapies (such as lapatinib or T-DM1) have not been successful, likely due to the fundamentally lower expression of HER2 in gastric carcinoma compared with breast carcinoma.
In the second-line setting, chemotherapy also has value, as both taxanes and irinotecan have shown an overall survival benefit in phase III trials (albeit with insufficient patient numbers). The anti-VEGFR2 antibody ramucirumab, as monotherapy versus placebo, also prolongs survival in patients with progression after first-line therapy [10]. This result was surprising because other VEGF antibodies showed no effect as monotherapy. Ramucirumab also prolonged overall survival in combination with paclitaxel (versus paclitaxel plus placebo) in a phase III trial (from 7.4 to 9.6 months, HR=0.807), so this agent is now approved alone and in combination with paclitaxel in Switzerland after pretreatment [11].
Immunotherapy
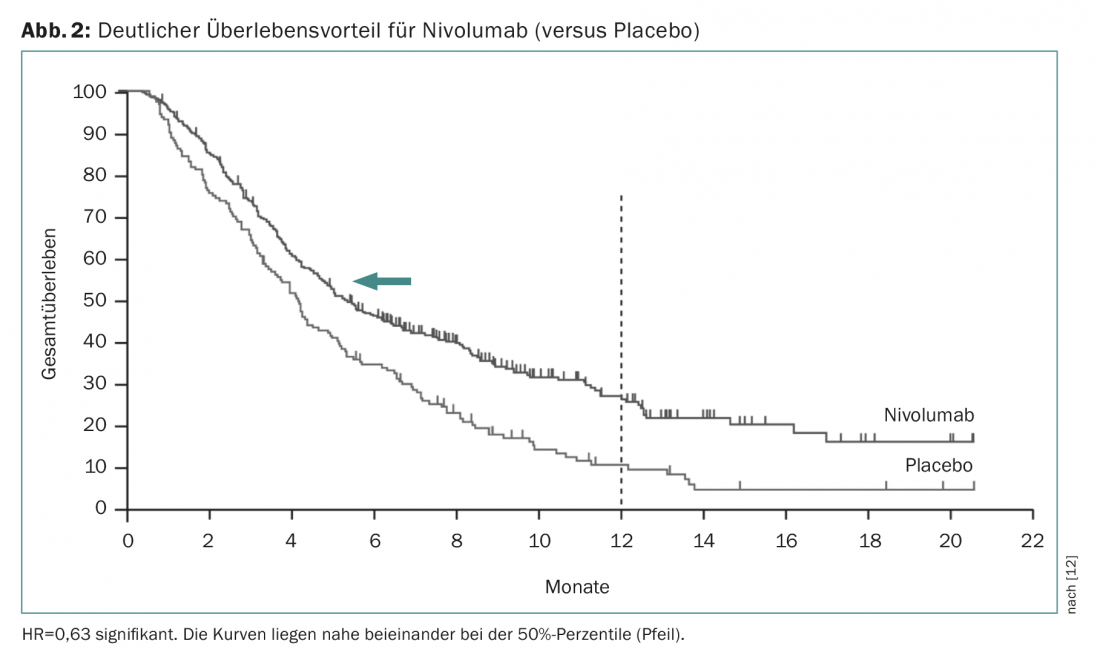
Nivolumab is a human monoclonal antibody (checkpoint inhibitor) that specifically binds to the PD1 receptor on activated T cells. In a phase III trial (Attraction trial), this treatment demonstrated a survival benefit from 4.1 to 5.3 months in pretreated patients compared with placebo [12]. The hazard ratio was much more significant (0.63, significant), showing that median survival times are strongly dependent on the progression of survival curves (Fig. 2) . Nivolumab is already approved in Japan for this indication. Since the studies were conducted with Asian patients and because the effect is essentially based on a small number of patients who respond well, it is currently still uncertain whether approval will also be granted in Switzerland. Prembrolizumab is another checkpoint inhibitor that is already widely used clinically. Based on a larger phase II study (KEYNOTE-059 study), this has now received the corresponding approval in the USA if PD-L1 expression can be detected in tumor tissue (at least in 1% of tumor or stromal cells). As a rule of thumb, 20% of patients can be expected to respond with checkpoint inhibition. Although fatal side effects occur, these immunotherapies are generally very well tolerated and produce noticeable sustained clinical improvement in the approximately 20% of patients in whom therapy results in a response.
Molecular testing
In the era of targeted therapies, the question arises as to what specific testing is currently useful in gastric/GEJ tumor. Apart from HER2 testing, microsatellite instability testing with regard to checkpoint inhibition certainly needs to be mentioned here. Although less useful as a predictive marker than microsatellite instability, as noted above, testing PD-L1 expression is useful with regard to checkpoint inhibition, particularly with regard to treatment with pembrolizumab. Despite intensive research, other molecular alterations are too rare or have no therapeutic consequences for clinical practice at the moment.
Summary
Surgery is the first choice for localized gastric carcinoma. In this regard, the use of systems therapy peri- or postoperatively can improve cure rates. Palliative systems therapy can prolong life and also improve quality of life.
Take-Home Messages
- In the curative situation, perioperative therapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) should be discussed with the patient starting at stage cT2.
- Palliative system therapy: In recent years, only ramucirumab has received approval as monotherapy or in combination. Checkpoint inhibition is clearly effective in a small proportion of patients. Microsatellite instability is by far the better predictive factor than PD-L1 expression.
Literature:
- Macdonald JS, et al: Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725-730.
- Al-Batran SE, et al: Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer. The AIO-FLOT3 Trial. JAMAOncology 2017; 3: 1237-1244.
- Noh SH, et al: Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 1389-1396.
- Lee J, et al: Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012; 30: 268-273.
- Cunningham D, et al: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11-20.
- Al-Batran SE, et al: Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. ASCO Annual Meeting 2017; Oral Abstract Session 1.
- Al-Batran SE, et al: The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited- metastatic adenocarcinoma of the stomach or esophagogastric junction – a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017; 17: 893.
- Al-Batran SE, et al: Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncology 2016; 17: 1697-1708.
- Bang YJ, et al: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687-697.
- Fuchs CS, et al: Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014 Jan 4; 383(9911): 31-39.
- Wilke H, et al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet 2014; 15: 1224-1235.
- Kang YK, et al: Nivolumab in patients with advanced gastric or gastroesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461-2471.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(3): 14-17.