Therapy of the disease with simultaneous fight against the “enemies” of the wound – a special role is played by bacteria and their interaction with the host. A good relationship with the primary care physician contributes to healing.
As recently as 15 years ago, a chronic wound was the fate of patients who filled doctors’ offices by frequent attendance. In recent years, a paradigm shift has taken place. Whereas at the time wound experts were a “rare species” who looked down from above on “unsuspecting general practitioners” and regarded only hand-picked surgeons as their equals, “wound ambis” have since mushroomed in hospitals and the private sector. The drive is the industry. There is now valuable continuing education for the “common doctor” species, so that modern wound care has found its way outside those conspiratorial chambers. The population is aging and treatment represents a significant economic factor. Last but not least, scientific research has helped to transform what were primarily purely nursing measures into sound, responsible and efficient medicine.
Causes
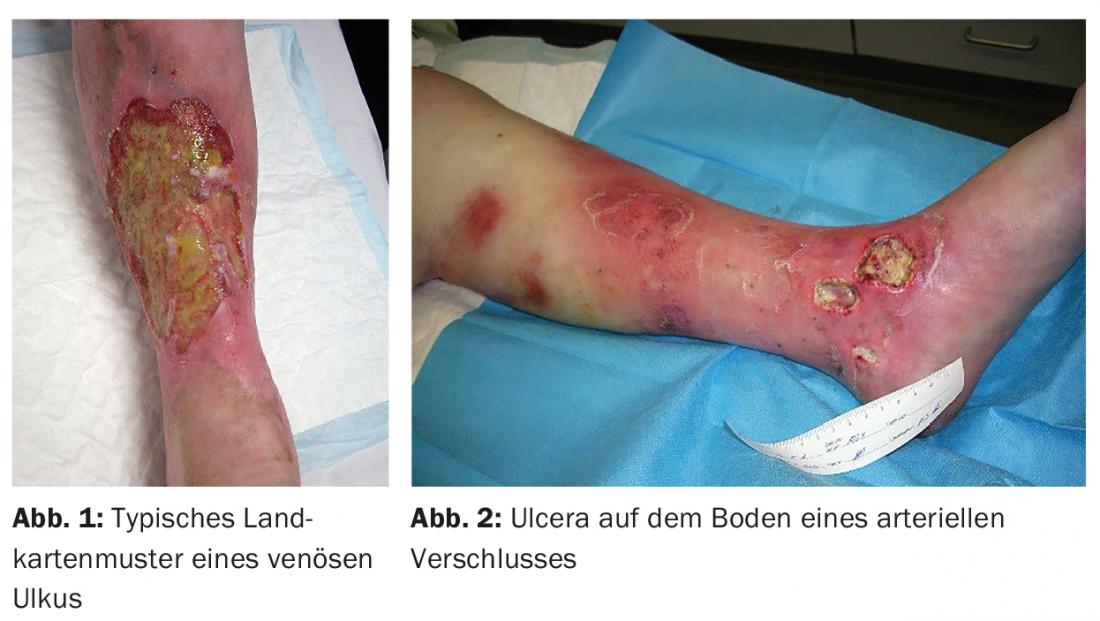
Chronic wounds in family practice usually have a vascular origin (in the context of chronic venous insufficiency, peripheral arterial occlusive disease, or not infrequently a combination of both) (Figs. 1 and 2) . Vascular complications frequently occur in the course of diabetes mellitus. Other causes are immunosuppressants, tumor diseases, malnutrition, neurological or general skin and connective tissue diseases.
Strategic approach
At the beginning of every treatment there is diagnostics. Knowing the cause of a wound is critical to treatment and prognosis. Vascular causes are detected with Doppler or angiography. Long-standing wounds require biopsy (as does suspected autoimmune disease). Pyoderma gangrenosum is a special case. The cause of this disease is not yet known for certain and it is always a clinical diagnosis. It’s a chameleon among wounds – you have to remember that. Treatment of pyoderma gangrenosum is with steroids and any debridement is to be omitted, in contrast to the treatment of all other wounds.
Why does a wound become chronic?
Even with the most careful diagnosis, targeted treatment of the underlying disease and therapy in accordance with the guidelines of modern wound treatment, the wound progresses to a point that can drive both patient and physician to despair.
The wound is a microcosm. It is not sterile, nor does it have to be. A balance of the bacteria on it does not cause major problems. However, the composition of the bacterial flora changes with the duration. Whereas aerobes were the main culprits for the time being, anaerobes are increasingly gaining the upper hand, starting from the poorly perfused deeper layers. Bacteria rot together, as they do at all boundary layers, be it in a wound, on an endoprosthesis or in our coffee machine. They form a consortium that covers itself with a layer of polysaccharides – the so-called biofilm, which is difficult to attack. Ultimately, they communicate when they want to break up the film again. In this way, they have something ahead of us. There is a release of bacteria and their toxins. This calls up neutrophil granulocytes. They provoke a release of proinflammatory cytokines and enzymes, and more proteases are secreted. There is a dysbalance of these factors, which leads to destruction at the wound edge of what is actually still healthy tissue. The alkaline pH value in the wound is a “feel-good climate” for bacteria. This enables them to produce bacterial enzymes, which in turn provoke the destruction of host tissue.
What can be done about it?
In no case are topical antibiotics suitable, as they eliminate only part of the bacteria. A dysbalance occurs, so that one bacterium gains the upper hand and triggers an infection. In case of infection, systemic antibiotic therapy is indicated. For simple infections, empiric cotrimoxazole is sufficient; prior to this, swab collection is recommended. For more severe infections, e.g., erysipelas, start wider with amoxicillin/clavulanic acid.
Local bacterial elimination is performed efficiently with an antiseptic. Before this, however, mechanical debridement should definitely be performed. The coating of bacteria, fibrin and necrosis must be removed. This can be done very well with a ring curette (Fig. 3). Local anesthesia is recommended. Emla® has a contact time of one hour. With Xylocain® Gel 2%, sufficient anesthesia is achieved within 15 minutes. In addition, the gel still softens the wound coating, which simplifies debridement. Both you need to cover with foil.

Wound Antisepsis
An overview is given in references [4] and [5]. Not every antiseptic is suitable. Antiseptics containing iodine, which have their place in the treatment of acute wounds, should not be used because of cytotoxicity. In chronic wounds, the focus is on efficacy against Gram-positive and Gram-negative bacteria, as well as tissue compatibility. Ringer’s or NaCl solutions alone are not capable of “cracking” a biofilm. Octenisept® is often available in the doctor’s office. The onset of action occurs within a few minutes. However, irrigation, especially in fistula tracts, is prohibited because it is toxic and can lead to severe necrosis. Flushing under pressure should also be warned against [6].
Polihexanide (Prontosan®) is also very effective against biofilm. It also promotes wound healing by increasing neoangiogenesis. However, it takes at least 30 minutes for the full effect to be achieved, so it is useful to moisten a dressing with polihexanide.
Highly effective are hypochloride solutions (OCl), which are non-toxic and control not only bacteria but also spores, aspergilli and enveloped viruses. The onset of action is faster and more efficient than with the previously mentioned antiseptics. OCl can also be applied with the dressing materials.
Honey has a strong antiseptic effect and stimulates wound healing. Only medicinal honey may be used (because of botulism). In some patients it causes a burning pain, but it subsides after a while.
Silver is assessed contradictorily. It destroys the cell wall of the bacteria and is thus bactericidal. However, some is always dissolved into the tissue and thus into the body, and it is considered toxic. One advantage of silver, honey and OCl solution is odor binding.
Hydrogen peroxide is still a popular remedy that is believed to have an antiseptic effect. However, it is immediately inactivated by endogenous peroxidases and also has an inhibitory effect on fibroblast proliferation, while the bacteria remain untouched.
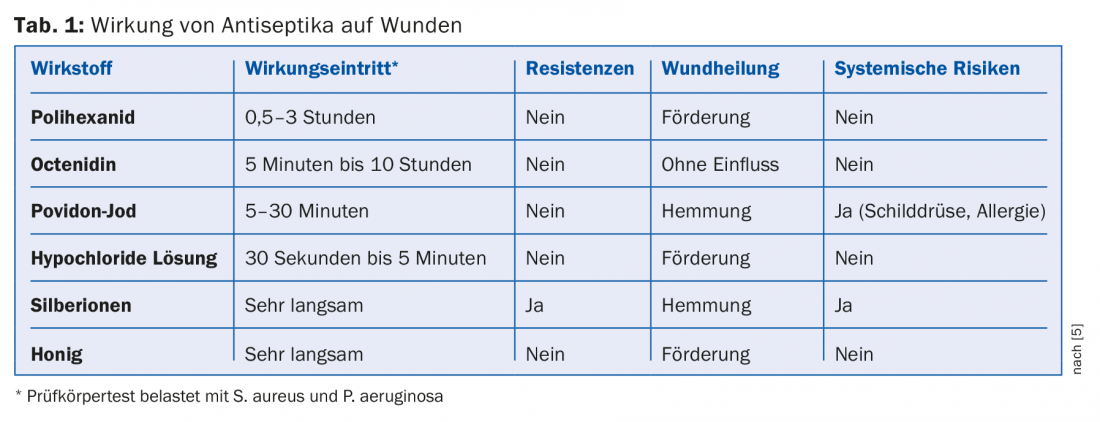
Table 1 summarizes again the effect of antiseptics on wounds.
The question of the association
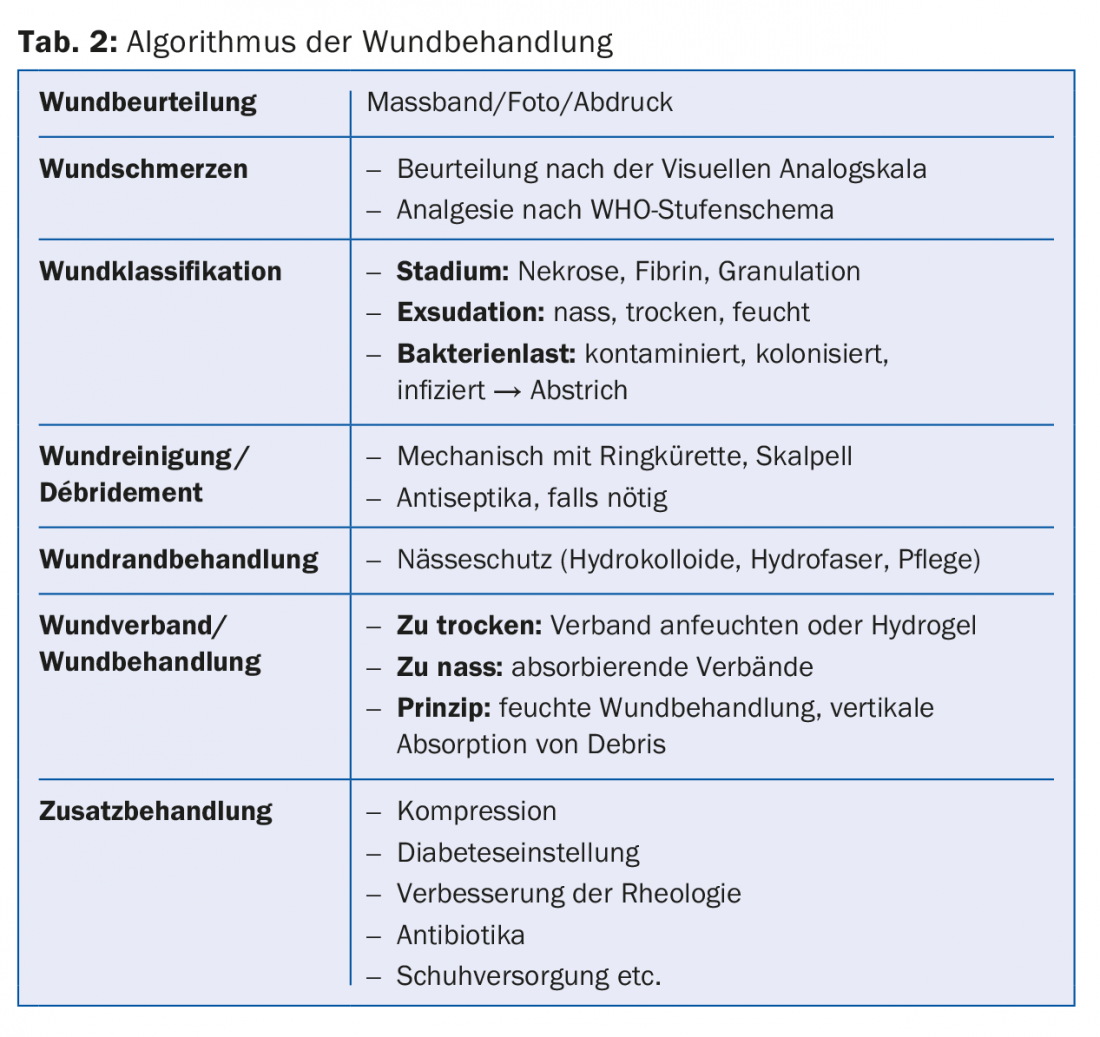
You think you can find an infinite number of different materials on the market. If you think about the principle of dressings, they all have something in common: absorption is vertical, so no debris is secreted back to the wound once it is caught in the dressing. The associations differ mainly according to the absorption capacity. Depending on the degree of dryness or exudation, semiocclusion with little evaporative capacity or greater absorption is needed. It is also important to tamp out wound cavities, e.g. with an alginate. Otherwise, all dressings can be applied directly to the wound. It is recommended to leave the dressing in place as long as the capacity of the dressing allows. If a wound needs to be checked daily, it can also be dressed with gauze (also moistened with the aforementioned antiseptics). Table 2 shows an algorithm of wound treatment.
And then there’s…
Adjuvant measures such as compression, reperfusion, adjustment of diabetes, pain management, nutrition, i.e. the creation of optimal conditions for wound healing are important.
Take-Home Messages
- Accompanying the therapy of the disease, the “enemies” of the wound are fought. Bacteria and their interaction with the host play a special role. Mechanical reduction of the biological load in combination with a suitable antiseptic is crucial.
- For the patient, integration into a social structure (doctor, family and other care institutions such as Spitex) is also very important.
- The patient is also a decision-maker in his or her own right and should actively participate in the process, which requires patience and good compliance with the mutually agreed path (“patient empowerment”) [1–3]. Passivity is a paralyzing factor. Confidence in the relationship with the primary care physician will contribute to healing.
Literature:
- Antonovsky A: Salutogenesis. Demystifying health. Dgvt Verlag 1997.
- Buchheim T: The responsible patient – five theses from the perspective of the normal consumer. Symposium of the Konrad Adenauer Foundation. 2005.
- Dietrich A: The modern patient – threat or promise. Patient Education and Counseling 2007; 67(3): 279-285.
- Kramer A: Wound antisepsis: evidence, indications, drug selection, and perspectives. Ars Medici 2016; 9: 419-426.
- Kramer A, et al: Wound antiseptics today – an overview. In: Willy C (ed.): Antiseptics in surgery – update 2013. Lindqvist 2013; 85-111.
- Gremingera M, et al: From minor injury to partial loss of function: on the inappropriate use of Octenisept® in hand injuries. Swiss Medical Forum 2016; 16(32): 642-644.
Further reading:
- Davies E: Honey in wound care: Theoretical and practical aspects for use in modern wound management. Departmental Work. Vienna 2006.
- Ott K, et al: Wound Manual Luzerner Kantonsspital Wolhusen/Sursee. 3rd revised edition. 2011.
- Knuf A: Empowerment Fördern – Beispiel Psychiatrie. Managed Care 2003 7; 17-19.
HAUSARZT PRAXIS 2017; 12(10): 8-11
DERMATOLOGIE PRAXIS 2018; 28(1): 11-14











