Early adequate therapy of rheumatoid arthritis (RA) is of central importance for the entire further course of the disease, current guidelines conclude. After diagnosis, the indication for disease-modifying therapy should be evaluated within three months. In addition to conventional synthetic DMARDs, a wide range of biologics and small molecule drugs are available today.
Rheumatoid arthritis (RA) is the most common of the approximately 400 rheumatological diseases. Typical symptoms of rheumatoid arthritis are pain, joint stiffness and restricted movement. Diagnosis is made based on history, the ACR/EULAR classification criteria, and other findings from inspection, laboratory, ultrasound, and radiographic examinations [1]. “The pattern of infestation is important,” said Konstantinos Triantafyllias, MD, Acute Rheumatology Hospital, Brad Kreuznach and University Medicine Mainz (D) [2]. The more smaller joints are affected, the more points there are according to ACR/EULAR classification criteria; a total score of ≥6 is considered rheumatoid arthritis. The temporal criterion is complaints existing for more than 6 weeks.
Rheumatoid factor often negative
Indicative laboratory findings for RA include [3]: elevated ESR, elevated CRP, detection of rheumatoid factors, and/or detection of antibodies to citrullinated peptides (ACPA/CCP-AK). However, in 50% of patients with incipient rheumatoid arthritis, the rheumatoid factor is undetectable. Conversely, a positive rheumatoid factor with increasing age occurs in up to 20% of healthy individuals. “Rheumatoid factor is often negative even in the early stages of rheumatoid arthritis, which is why we need other markers,” Dr. Triantafyllias summarized [2]. CCP antibodies, also known as ACPA, are an important additional marker that has high specificity for rheumatoid arthritis. Whereas recent studies have reported an increasing incidence of ACPA-negative RA [4,5].
“Optical Spectral Transmission Technology” – new process
Erosions visible on x-ray are usually not apparent until a later stage of the disease, the speaker said. One should also think of the cervical spine, this is also circumscribed as the fifth limb of the rheumatic. Instability of the atlantoaxial joint threatens the spinal cord.
Sonographic examinations also play an important role. One can puncture fluid and perform laboratory diagnostics to more accurately determine disease activity. Also important is MRI with or without contrast, both for initial diagnosis and for follow-up assessment. In addition, there are some exciting new developments in the field of imaging diagnostics, such as “Optical Spectral Transmission Technology”. This is a noninvasive laser-based imaging technique that visualizes blood flow in the area of swollen joints, explains Dr. Triantafyllias [2]. Moreover, quantification is possible. One study demonstrated correlations with clinic (DAS28-CRP), inflammatory markers (CRP), and arthroscopic findings [6].
Symptomatic therapy is important, but not disease modifying
In an acute attack, NSAIDs/ coxibs and/or cortisone preparations are often initially used for symptomatic therapy. Although these substances are an important part of treatment in that they have analgesic and/or anti-inflammatory effects, they do not influence disease progression or joint destruction. According to the EULAR guideline, cortisone preparations should generally not be used for longer than three to six months in rheumatoid arthritis.
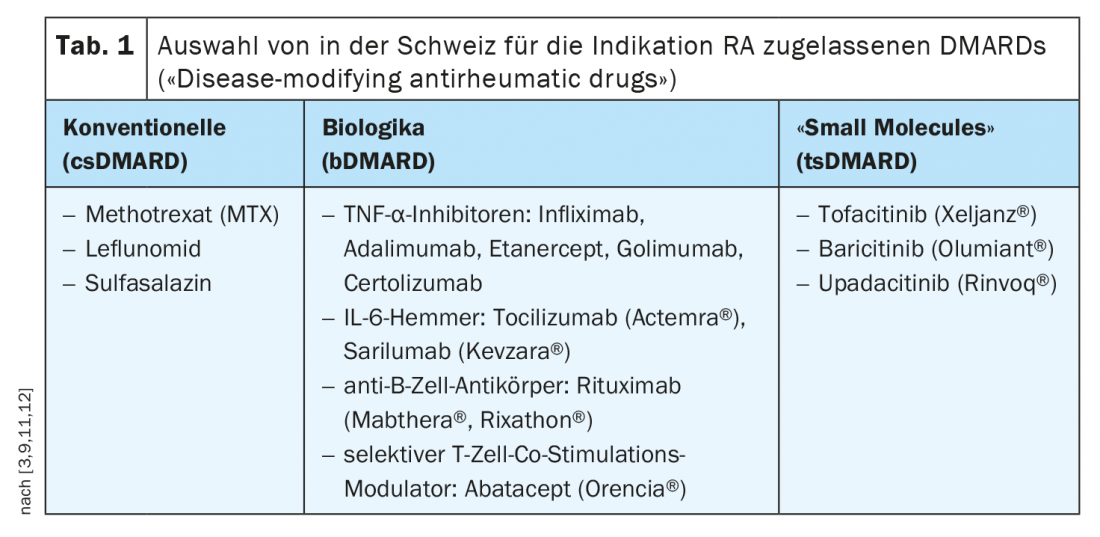
In contrast, DMARDs are drugs that have a long-term positive effect on the course of rheumatoid arthritis or other chronic inflammatory rheumatic diseases by halting or at least slowing the progression of the disease. Disease-modifying drugs are divided into conventional synthetic agents (csDMARDs), biologics (bDMARDs), and targeted synthetic base drugs (tsDMARDs).
Conclusion of the guidelines: Do not miss the window of opportunity for DMARDs
Recent studies have shown that rapid control of disease activity by early initiation of therapy with disease-modifying agents is critical for further prognosis [7,8]. To take advantage of this window of opportunity, both the guideline “Therapy of Rheumatoid Arthritis with Disease Modifying Drugs” and the “Interdisciplinary Guideline Management of Early Rheumatoid Arthritis” recommend initiating disease modifying (DMARD) therapy within three months of diagnosis [3,9]. The motto is that early use of DMARDs has a favorable effect on prognosis, taking into account individual patient characteristics and situational factors. Implementation of these treatment recommendations requires coordinated interdisciplinary care in which the initial disease signals are correctly interpreted, the indication for disease-modifying therapy is assessed, and affected individuals are promptly referred to a rheumatologist.
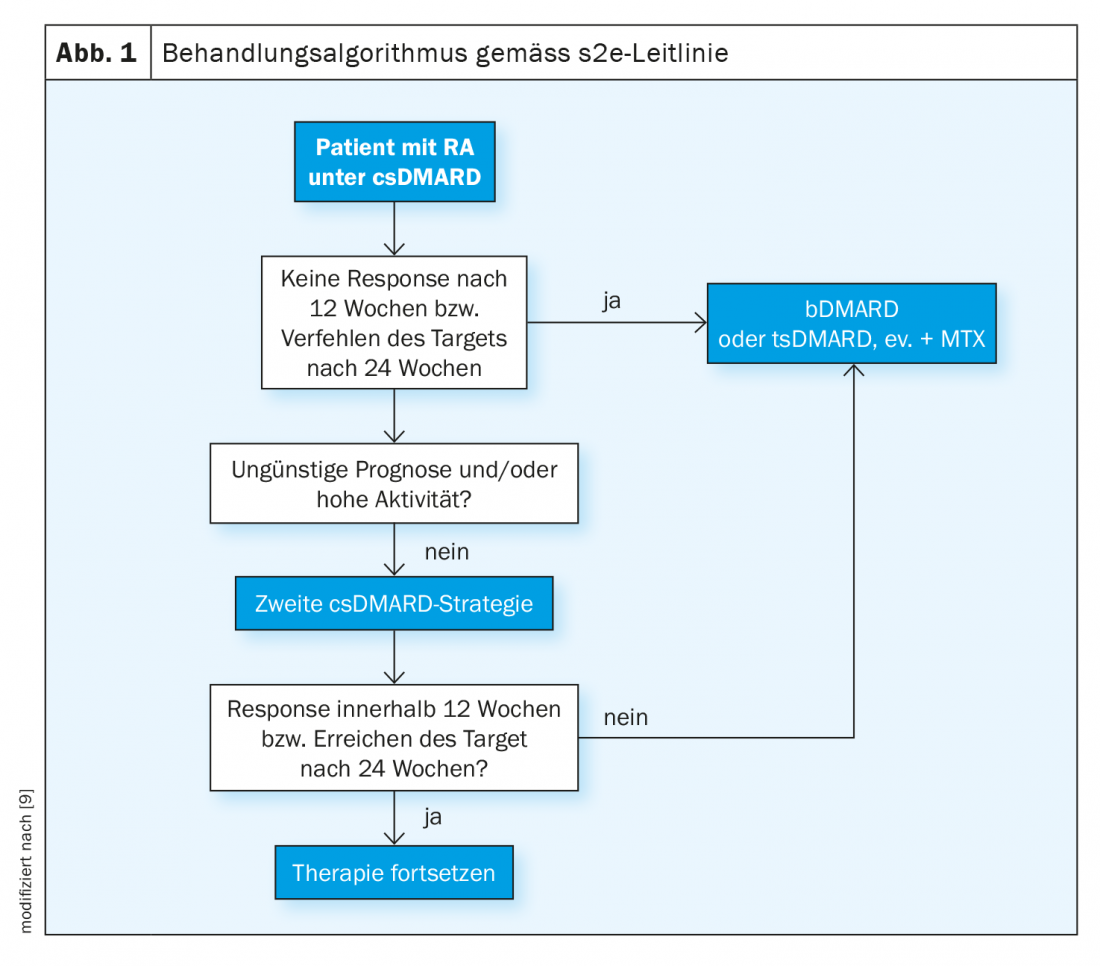
When to switch from csDMARDs to biologics or “small molecules”?
The s2e guideline recommends using methotrexate first if there are no contraindications (e.g., renal insufficiency). Alternatively, leflunomide and sulfasalazine can be used [9]. It is best to initially combine csDMARDs with glucocorticoids, as it sometimes takes 6-12 weeks to achieve a response to therapy. The default is up to 30 mg prednisolone equivalent daily, with a dose reduction to “low dose” (e.g., 5 mg or 7.5 mg) within 8 weeks. Parallel to the induction therapy, a progress control is to be carried out. When is someone a responder? According to the guideline, if the composite score of disease activity (e.g., DAS28) improves by 50% three months after initiation of therapy. The DAS28 records the number of pressure painful and swollen joints, as well as the subjectively perceived disease activity and inflammation scores. If a 50% reduction in DAS28 has been detected, therapy can be continued with the goal of remission (0-1 swollen or tender joints). “If this is not achieved, therapy escalation must take place,” the speaker explained [2]. In this case, this means switching to biologics therapy (Fig. 1) . This is especially true if prognostically unfavorable factors are present such as erosions, high disease activity and seropositive findings. “Then you shouldn’t waste a lot of time with a second conventional synthetic DMARD,” the speaker said. After 3-6 months, the response to therapy is monitored.
“Treat-to-Target” is the motto
If there is insufficient response or intolerance to initial bDMARD therapy, switching to an alternative bDMARD with the same or different mechanism of action or to a tsDMARD is recommended. If therapy after csDMARDs is started with a tsDMARD instead of a bDMARD, a switch to a biologic should be made if possible if there is no response. Targeted synthetic basic drugs (tsDMARDs, targeted synthetic Disease Modifying Anti-Rheumatic Drug) are the newest subset of basic drugs. Unlike biologics, tsDMARDs do not intercept proinflammatory messengers in the blood, but act within cells to interrupt the signaling pathway that promotes inflammation. The efficacy of JAK inhibitors in rheumatoid arthritis is comparable to that of biologics.
Congress: DGIM Annual Conference 2021
Literature:
- Aletaha D, et al: 2010 Rheumatoid Arthritis Classification Criteria, Arthritis & Rheumatism 2010; 62(9): 2569-2581.
- Triantafyllias K: Diagnostics and primary therapy for the internist in rheumatoid arthritis. Konstantinos Triantafyllias, MD, DGIM Annual Meeting, April 19, 2021.
- Schneider M, et al: S3 guideline: interdisciplinary guideline management of early rheumatoid arthritis, 2019, AWMF registry no. 060/002.
- Myasoedova E, et al: Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Annals of the Rheumatic Diseases 2020; 79: 440-444.
- Matthijssen XME, et al: Increasing incidence of autoantibody-negative RA is replicated and is partly explained by an aging population. Ann Rheum Dis 2020, doi: 10.1136/annrheumdis-2020-217609.
- Triantafyllias K, et al. Diagnostic Value of Optical Spectral Transmission in Rheumatoid Arthritis: Associations with Clinical Characteristics and Comparison with Joint Ultrasonography. J Rheumatol. 2020; 47(9): 1314-1322.
- Combe B, et al: 2016 update of the eular recommendations for the management of early arthritis. Annals of the rheumatic diseases 2017; 76: 948-959.
- van Nies JA, et al.: Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: Does a window of opportunity exist? Results on the leiden early arthritis clinic and espoir cohorts. Annals of the rheumatic diseases 2015; 74: 806-812.
- Fiehn C, et al: S2e guideline: therapy of rheumatoid arthritis with disease-modifying drugs, 2018, AWMF Register Number: 060-004.
- Deutsche Rheuma-Liga Bundesverband e.V., www.rheuma-liga.de/rheuma/therapie/medikamententherapie/schmerzmedikamente (last accessed 16.06.2021)
- Rheumaliga Schweiz: Rheumatoid Arthritis: Living with a Chronic Disease, www.rheumaliga.ch/assets/doc/ZH_Dokumente/Broschueren-Merkblaetter/Krankheitsbilder/RA.pdf, (last accessed 16.06.2021)
- Swiss Society of Rheumatology: Recommendation Basic Therapy 2021.
HAUSARZT PRAXIS 2021; 16(7): 26-27 (published 6/27-21, ahead of print).