In the field of rare diseases, there is an enormous need to catch up in terms of knowledge, correct diagnosis and therapy. Many primary care physicians are also feeling this right now. After all, what general practitioner would think of light chain (AL) amyloidosis when the patient comes to the office with symptoms that would be diagnosed as mild heart failure on the face of it?
Unfortunately, it often takes a long time until an adequate diagnosis of amyloidosis is made. Patients feel this firsthand because the complaints are steadily increasing. Light-chain amyloidosis is a rare disease and the symptoms are unclear, so that even specialists cannot always classify them immediately. However, because AL amyloidosis is progressive and organ damage increases, rapid and correct diagnosis is central.
Epidemiology
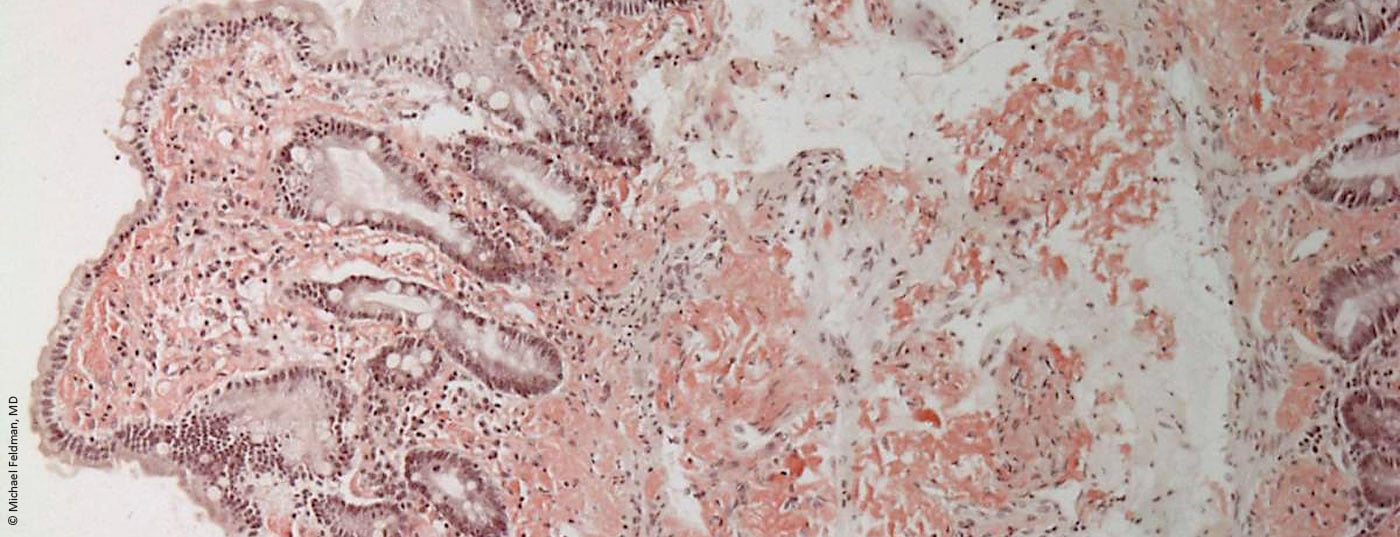
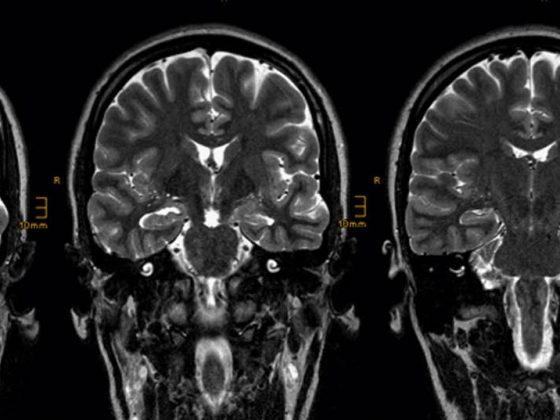
In industrialized countries, AL amyloidosis is the most common form of amyloidosis. Triggers are pathologically increased plasma cells or, rarely, lymphocytes. The antibodies produced as a result are defectively formed in one of the light chains. These therefore clump together and the resulting amyloid is deposited as poorly soluble fibrils in organs. The heart muscles, gastrointestinal tract, kidneys, liver and spleen are particularly often affected. However, unusual tongue swelling along with heart failure may also provide a clue for a more specific diagnosis.
There is a risk of misdiagnosis
Causes of AL amyloidosis include monoclonal gammopathies, previously diagnosed malignant blood disorders, or lymph node disease. However, it can also occur without such and therefore often remains undetected for a very long time in older people. This is also because the classic symptoms, such as shortness of breath, dysrhythmia or edema in the legs, may initially lead to a different diagnosis. However, if additional symptoms such as low blood pressure, weakness, foamy urine, upper abdominal discomfort, numbness or tingling and even stabbing pain, an enlarged tongue and bleeding in the skin around the eyes appear, it is imperative that clarification by a specialist be initiated.
What therapies are available?
Therapeutically, there are so far only limited options for the oncologist. These include moderate to severe chemotherapy in combination with cortisone or stem cell therapy aimed at destroying the bone marrow with reduction of plasma or lymphocytes and antibodies. The severity of chemotherapy here depends on the patient’s age, general condition, and organ resilience. Thalidomide is also used for therapy. However, there is also a potential for side effects here.
Unfortunately, often the side effects of chemotherapy are so severe that patients discontinue it because of them. What other therapies will be available in the future is still uncertain. However, various competence centers are involved in research projects. In a few cases, a few of the symptoms could be alleviated. However, the lost kidney function cannot be restored. Therefore, rapid diagnosis and possible therapeutic intervention is critical to the patient’s quality of life.
In addition to these attempts to halt AL amyloidosis, supportive treatment should be given to the affected organ systems. Here, the informed family doctor takes over the further therapy.
Hopefully, with the increase in knowledge about rare diseases, primary care physicians will be better equipped to consider amyloidosis as a differential diagnosis in the future.
Share and discuss your experiences: Orphanbiotec blog under the topic amyloidosis.
www.blog.orphanbiotec-foundation.com
HAUSARZT PRAXIS 2014; 9(7): 55-56