Since the PARADIGM-HF study, angiotensin receptor neprilysin inhibitors, or “ARNIs”, have been the talk of the town: a breakthrough in the treatment of heart failure, a milestone or a quantum leap. In the meantime, it has also become clear where the “new kid on the block” valsartan/sacubitril – if the new ESC guidelines are anything to go by – should be located in the therapy algorithm. Are the Europeans taking a similar path to the Americans and de facto recommending the new substance at the first-line level? At the joint annual meeting of SGK, SGHC and SGP in Lausanne, Prof. Frank Ruschitzka, MD, President Elect of the Heart Failure Association (HFA) of the ESC, summarized the innovations in a well-attended Main Session.
“2.1% of the Swiss population, or a total of 175,000 people, suffer from heart failure – and the trend is rising,” Prof. Ruschitzka said by way of introduction. “Although this is likely to be a conservative estimate.” In recent years, the disease has increasingly become the focus of attention. Far ahead of acute myocardial infarctions and cancers such as prostate and breast, it is the most common reason for hospitalizations. The good news is that treatment, at least for systolic heart failure, has evolved in recent years from a primarily palliative approach to a life-saving therapy. Notable among these are ACE inhibitors, beta-blockers, and mineral corticoid receptor antagonists (MRAs) with their beneficial effects on mortality [1]. “Today, we are increasingly dealing with a chronic disease where we follow patients for years.”
New on the stage of mortality-lowering therapeutics is ARNI, the “shooting star” among heart failure drugs. The associated trial called PARADIGM-HF [2] was stopped due to the clear superiority of LCZ696 (ARNI) over enalapril [2]. In this study, 8442 patients with NYHA class II-IV and an ejection fraction equal to or less than 40% had received either LCZ696 (200 mg twice daily) or enalapril (10 mg twice daily) in addition to previous best therapy. Compared with enalapril, LCZ696 significantly reduced all-cause mortality risk (secondary endpoint) by 16% (17.0 vs. 19.8%) and the primary endpoint, a composite of cardiovascular-related deaths and heart failure (HI) hospitalizations, by 20% (21.8 vs. 26.5%, p<0.001).
When is ARNI recommended?
“Today, we are in the comfortable situation that we have access to many therapy options. But the more drugs we have available, the more complex the therapy algorithm becomes,” said Prof. Ruschitzka, summarizing the new recommendations.
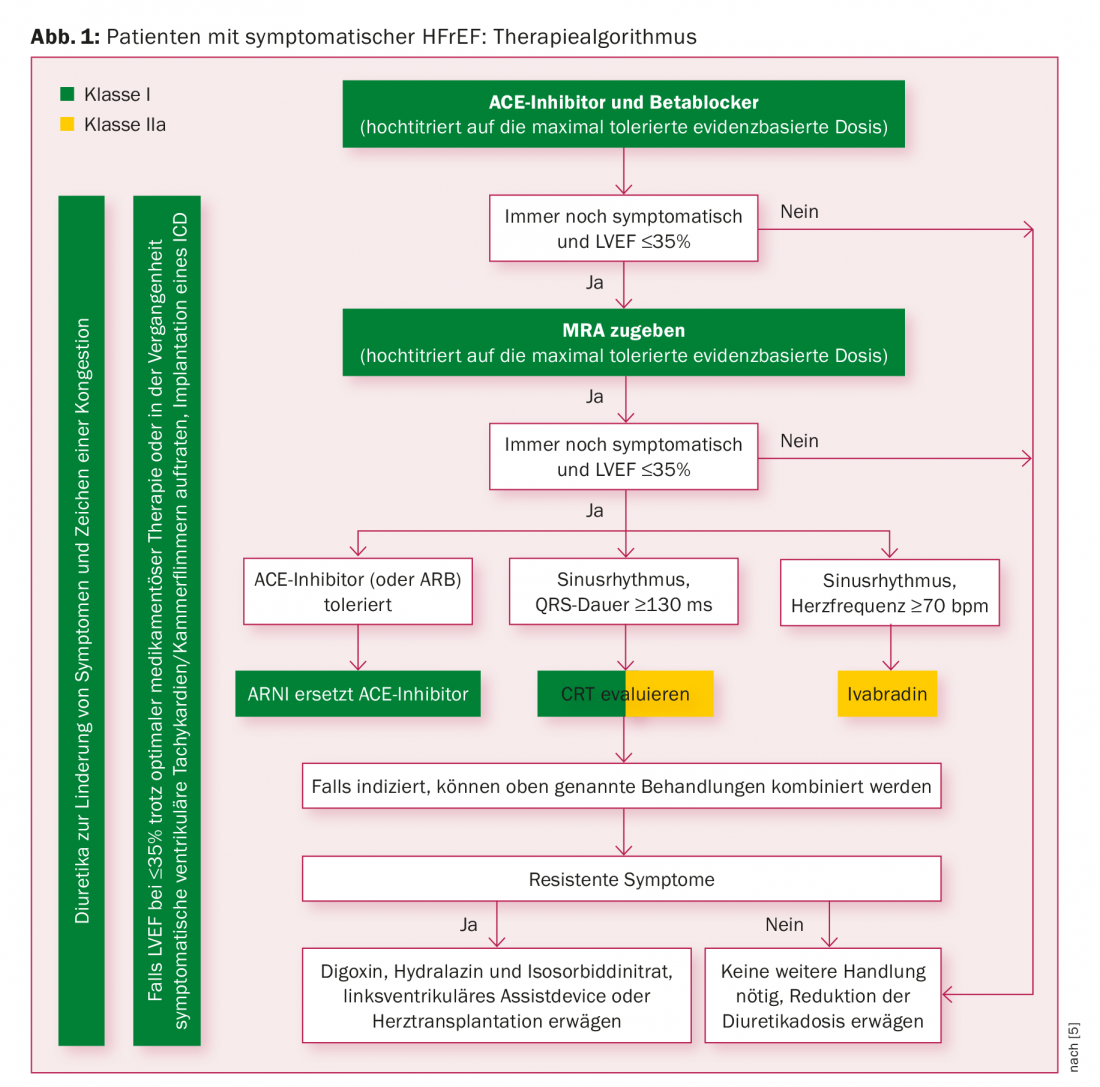
The update of the US guidelines de facto elevates ARNI to the rank of first-line therapy by recommending either ACE inhibitors or AT1 receptor blockers or just ARNI for morbidity and mortality reduction in HI patients with reduced ejection fraction (HFrEF) and concomitant beta-blocker (and MRA) use (class I recommendation, for ARNI IB, for the others IA); the European guidelines published at the same time are more cautious [3]. The base remains ACE inhibitors – AT1 receptor blockers (ARBs) if intolerant – and beta blockers, titrated up to the maximum tolerated evidence-based dose. If patients remain symptomatic with a left ventricular ejection fraction (LVEF) of ≤35%, MRA is added. Only if this measure does not bring about a change should an ARNI replace the ACE inhibitor (only in patients who have already tolerated an ACE inhibitor). This recommendation has the status IB. The corresponding therapy algorithm is shown in Figure 1.
Prof. Ruschitzka also reminded again of the most important contraindications (known history of angioedema, severe renal dysfunction with eGFR <10 ml/min/1.73 m2, pregnancy) when dealing with valsartan/sacubitril. Valsartan/sacubitril must be administered no earlier than 36 hours after discontinuation of ACE inhibitor therapy and must not be combined with an ACE inhibitor, aliskiren, or an ARB. Caution should also be exercised regarding hyperkalemia and hypotension. The same is true for patients with an eGFR between 10 and 30 ml/min/1.73 m2.
An initial dose of 2× 50 mg/d is recommended in patients previously treated with a low dose of ACE inhibitors or ARBs. Otherwise, the recommended initial dose of Entresto® is 2× 100 mg/d with an increase every two to four weeks to the target dose of 2× 200 mg/d.
Is there an “intermediate” form of heart failure?
The second innovation in the ESC guidelines, which will certainly be discussed intensively in the future, is the introduction of a “middle class” of HI: The HFmrEF (Heart Failure mid-range Ejection Fraction) lies classificatory between systolic (HFrEF) and diastolic HI (HFpEF). The new group thus enters the gray zone between HFrEF with an LVEF of <40% and HFpEF with an LVEF of ≥50%. Thus, in this “intermediate” form, the LVEF is 40-49%. Whereas in HFrEF, clinic (symptoms with/without signs of disease) and reduced ejection fraction are sufficient for diagnosis, in HFpEF and HFmrEF, natriuretic peptide levels must be elevated and additional findings must be present that demonstrate structural or functional damage to the myocardium. The new classification is summarized in Table 1.

CRT – contraindication with QRS duration <130 ms
Cardiac resynchronization therapy (CRT) is recommended for symptomatic patients with an ejection fraction ≤35%, a QRS duration ≥150 ms, and left bundle branch block with status IA when symptoms persist despite optimal medical therapy. “Here the case is clear,” Prof. Ruschitzka summarized the study situation. “A QRS duration <130 ms is now clearly a CRT contraindication after the negative results of the EchoCRT trial [4]. What happens in the area in between? Further clarification is needed here; CRT currently receives an IB recommendation in patients with left bundle branch block.”
What about diastolic heart failure?
To date, no treatment has demonstrated a convincing reduction in morbidity or mortality in patients with HFmrEF and HFpEF. Therefore, screening and treatment of cardiovascular and non-cardiovascular comorbidities, which are usually found in large numbers in this group, remain of great importance. Important comorbidities include hypertension, atrial fibrillation, diabetes, and ischemia. Diuretics can improve the symptoms and signs of disease in congestive
Alleviate HFmrEF and HFpEF patients.
“Principles for treating comorbidities in general are: Treat iron deficiency; metformin as first line in diabetes plus HI; in hypertension, add diuretics, amlodipine, or felodipine for optimal drug therapy of HFrEF,” Prof. Ruschitzka explained.
Source: Joint Annual Meeting SGK, SGHC, SGP, June 15-17, 2016, Lausanne.
Literature:
- McMurray JJ: CONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin-angiotensin-aldosterone system the cornerstone of therapy for systolic heart failure. Eur J Heart Fail 2011 Sep; 13(9): 929-936.
- McMurray JJ, et al: Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014 Sep 11; 371(11): 993-1004.
- Yancy CW, et al: 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016 May 20. DOI: 10.1161/CIR.00000000000435. [Epub ahead of print].
- Ruschitzka F, et al: Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013 Oct 10; 369(15): 1395-1405.
- Ponikowski P, et al: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. EHJ 2016, May 20. DOI: http://dx.doi.org/10.1093/eurheartj/ehw128 [Epub ahead of print].
CARDIOVASC 2016; 15(4): 25-27