From the patient’s point of view, a rapid onset of action is one of the most important treatment goals, along with appearance-free skin. Both the data from the UNCOVER-3 study and head-to-head studies demonstrate the rapid onset of symptom relief with ixekizumab in moderate to severe psoriasis. As longitudinal data show, the high efficacy with continuous treatment persists after several years.
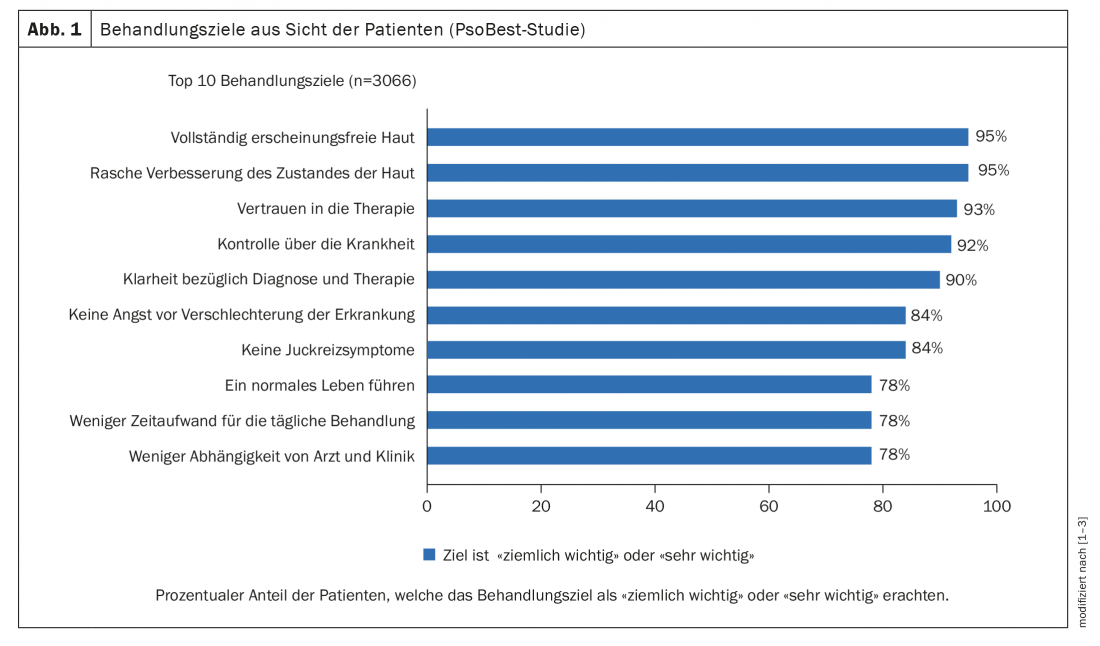
Data from the PsoBestGermany study, in which more than 3,000 patients were surveyed, show that for adult patients with moderate to severe psoriasis, one of the most important treatment goals, in addition to lesion-free skin, is to achieve symptom relief as quickly as possible. [1,2] (Fig. 1). The question of the extent to which a rapid therapeutic response is associated with long-term efficacy is being investigated in longitudinal studies. UNCOVER-3 is a randomized, double-blind, multicenter, phase III efficacy and safety study of ixekizumab (Taltz®) [8]. The results show that the extent of symptom reduction at week 12 correlates with quality of life (DLQI), explains Andreas Pinter, MD, Department of Dermatology, Venereology and Allergology, University Hospital Frankfurt am Main (D) [1]. The proportion of patients with a DLQI 0/1 is significantly higher in patients with a PASI100, PASI90, or PASI75 compared with those with lower PASI scores. “The results of ixekizumab at week 12 are very good not only in terms of relief of severity, but also in terms of improvement of quality of life and this is very important for the patients,” Prof. Uwe Gieler, MD, Psychosomatic Dermatology, University Hospital Giessen (D), summarized these data [3].
Early treatment response is associated with long-term efficacy
A network meta-analysis published in 2020 shows that the new generation of biologics has major advantages in terms of duration to onset of action compared to earlier representatives of these systemic therapeutics [4]. The IL-17A inhibitor ixekizumab is among the two biologics with the most rapid onset of action in this analysis. After only 12 weeks, patients with moderate to severe psoriasis showed a significant improvement in skin condition and quality of life. As shown in an analysis of data from the UNCOVER-3 trial published this year, the high efficacy of ixekizumab persists over a 4-year period with continued treatment. “When patients show a rapid response, there is an increased chance that the high efficacy will last for a longer period of time,” Dr. Pinter explained [1]. As shown in a subgroup analysis of the UNCOVER-3 trial, PASI-50 scores at week 2 after a treatment period of 3 years are associated with both better effects and a lower treatment discontinuation rate [5]. Further evaluations of the UNCOVER-3 study also demonstrate that ixekizumab also provides rapid and sustained relief of nail infestation. After 4 years of continuous ixekizumab treatment, a proportion of 67% of patients had a score of 0 on the Nail Psoriasis Severity Index (NAPSI).
|
PASI values correlate with quality of life and compliance Evaluations of the UNCOVER-3 trial show that a rapid response to ixekizumab treatment is associated with an improvement in quality of life [7]. This is also important because moderate to severe psoriasis is often associated with a significant “burden of disease” and psychosocial impairment. This in turn can have a negative impact on compliance. Rapid symptom relief also increases patients’ motivation for treatment and thus the chance of sustained therapeutic success [1,3]. |
Ixekizumab also performed extremely well in head-to-head studies in terms of efficacy at 12 weeks and was shown to be superior to etanercept (UNCOVER-2, UNCVOVER3), ustekinumab (IXORA-S), and guselkumab (IXORA-R) in PASI100 response rates [1]. In the head-to-head IXORA-R trial, ixekizumab was found to be significantly superior to guselkumab at weeks 7, 8, and 12 and non-inferior to guselkumab at week 24 in terms of PASI100 response rates** [6]. “Nearly a quarter‡ of patients treated with ixekizumab show a PASI-75 response after only 2 weeks,” Prof. Gieler emphasized [3].
Conclusion
As the long-term data of the UNCOVER-3 study show, a rapid response to therapy is not only in line with patients’ wishes, but is associated with sustained high efficacy and a low treatment discontinuation rate. The improvement in quality of life associated with the relief of cutaneous symptoms is of high relevance to the daily lives of patients, who often suffer from stigmatization and comorbid mental disorders (box). The rapid onset of action of ixekizumab was confirmed in head-to-head studies. The safety profile is consistent with previous studies, and no new safety signals have been reported [1,7]. Ixekizumab (Taltz®) is approved in Switzerland for the treatment of moderate to severe plaque psoriasis when other systemic therapies are intolerant or contraindicated [8].
* PBI-S Patient Needs Questionnaire at initiation of new system therapy.
** non-responder imputation, NRI analysis.
‡ 23%, according to non-responder imputation (NRI) analysis in the Ixekizumab 80 mg q2w condition (n=520).
Literature:
- Pinter A: Complete and sustained skin clearance or rapid onset in psoriasis: Should this be a choice? Andreas Pinter, MD, Clinic for Dermatology, Venereology and Allergology, University Hospital Frankfurt am Main (D), Webinar, Eli Lilly, October 2020.
- Blome C, et al: Patient-relevant treatment goals in psoriasis. Arch Dermatol Res 2016; 308(2): 69-78.
- Gieler U: Implications of rapid onset for patient treatment satisfaction and clinical outcomes. Prof. Dr. med. Uwe Gieler, Department of Dermatology, Justus-Liebig University Giessen (D), Webinar, Eli Lilly, October 2020.
- Warren RB, et al: Rapid response of biological treatments of moerate-to-severe plaque psoriasis : A comprehensive investigation using Bayesian and frequential network meta-analyses. Dermatol Thera 2020; 10(1): 73-86.
- Rosmarin D, et al: Presented at the American Academy of Dermatology (AAD) Virtual Meeting Experience (VMX), www.aad.org/member/meetings-education/aadvmx
- Blauvelt A, et al: A head-to-head comparison of ixekizumab vs guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol 2020; 182(6): 1348-1358.
- Lebwohl MG, et al: Ixekizumab sustains high level of efficacy and favorable safety profile over 3 years in patients with moderate psoriasis: Results from UNCOVER-3 study. JEADV 2020; 34(2): 301-309.
- Subject information, www.compendium.ch
DERMATOLOGY PRACTICE 2020; 30(6): 40-41