The Federal Office of Public Health (FOPH) and the Swiss Federal Commission for Immunization Questions (EKIF) recommend vaccination against herpes zoster with the new recombinant inactivated vaccine consisting of the two active ingredient components glycoprotein E (virus subinit) and AS01B (adjuvant) for healthy persons aged 65 years and older and for patients with immunodeficiency aged 50 years and older or with severe immunodeficiency aged 18 years and older. This is now approved for cash and available in Switzerland.
With age, the number and functionality of immune cells that prevent reactivation of the varicella-zoster virus (VZV) decrease [2,6,7,9,10]. This leads to an increase in the incidence and severity of shingles. In Switzerland, there are between 17,000 to 30,000 cases of herpes zoster annually [2]. Approximately 99% of adults 50 years of age and older are infected with the shingles-causing virus, and in one in three individuals, latent VZV reactivates and causes shingles [2,3]. Certain pre-existing conditions such as rheumatoid arthritis, inflammatory bowel disease, diabetes, COPD, asthma, systemic lupus erythematosus, or depression also lead to an increased risk of herpes zoster. The risk of herpes zoster is highest in diseases that lead to severe immunodeficiency, such as lymphoma or myeloma, and the risk is increased 13-fold during chemotherapy [2,5,6,11,12].
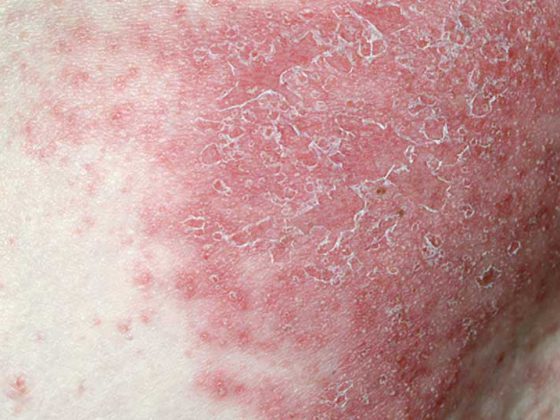
Shingles and the potentially serious complications.
Shingles is a painful disease that can be associated with serious and long-lasting complications, which include postherpetic neuralgia (PHN) and zoster ophthalmicus [2]. PHN affects up to 30% of shingles patients and is characterized by nerve pain, sometimes lasting for years [2,4]. Zoster ophthalmicus affects about 20% of shingles patients. This can lead to ophthalmologic complications and, in rare cases, blindness [2,13]. The herpes zoster recurrence rate after eight years is estimated at 6.2% according to Yawn et al. Among them, recurrences were more frequent in individuals with pain lasting >30 d and in immunosuppressed individuals. Because of the relatively high recurrence rates, vaccination should also be offered to patients who have already suffered from herpes zoster [14].
Prevention through new recombinant inactivated vaccine
SHINGRIX® is the first and only shingles vaccine to induce a strong, sustained immune response using a combination of a recombinant antigen and an adjuvant system [1,15–19]. The antigen (glycoprotein E [gE]) triggers a specific immune response against VZV. gE is expressed on the surface of VZV-infected cells and is critical for virus replication. The adjuvant system (AS01B) causes an enhancement of the immune response to the vaccine antigen. It induces a strong, sustained antigE immune response. The unique combination of MPL and QS-21 enhances both humoral and cellular immune responses against gE* [1,17–22]. This property also makes it suitable for immunodeficient or immunosuppressed patients [1].
* VZV = varicella zoster virus; MPL: 3-O-desacyl-4′-monophosporyl lipid A of Salmonella minnesota; QS-21: saponin fraction QS-21 of soap bark tree.
Efficacy and safety profile
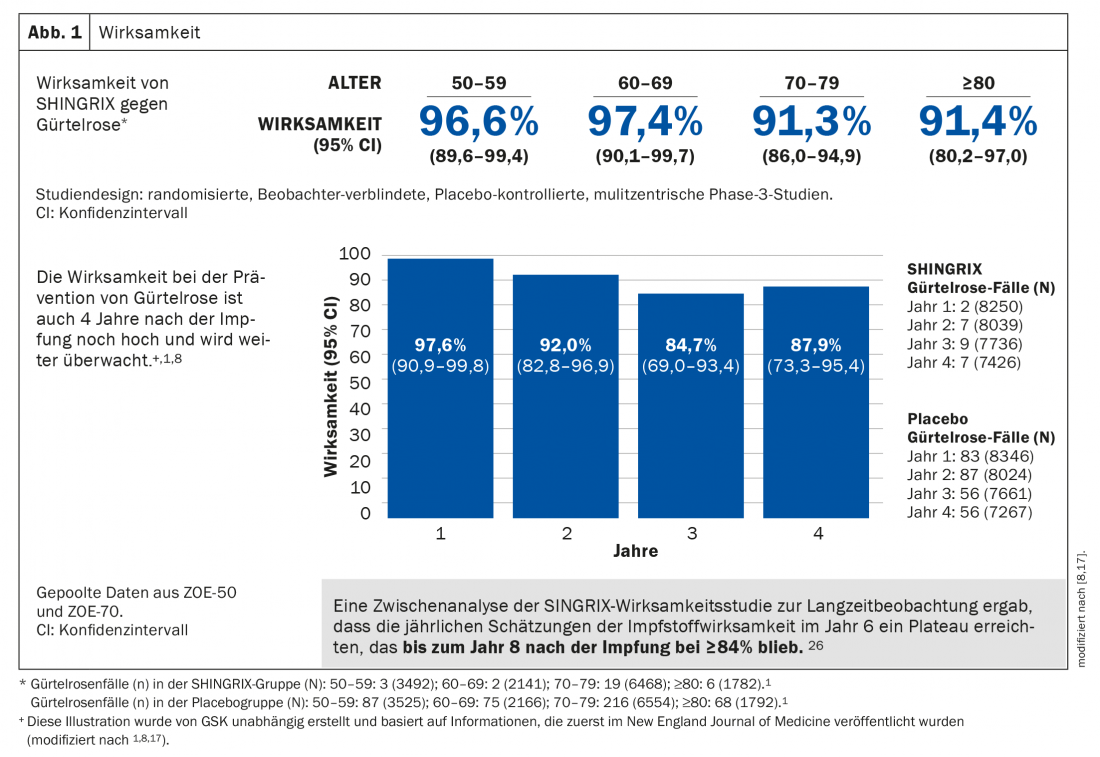
The adjuvanted subunit vaccine demonstrated greater than 90% efficacy in preventing shingles in all age groups 50 years and older, remaining at very high levels after more than seven years. Likewise, the risk of developing PHN is significantly reduced (Fig. 1) [1]. Safety has been extensively evaluated in two large-scale Phase 3 clinical trials. The majority of adverse events were mild to moderate in severity and included pain, redness, and swelling at the injection site. Patients may also suffer from muscle pain, fatigue, headache, chills, fever or upset stomach. In clinical trials, these adverse effects lasted a median of 2 to 3 days [1,8,17].
Vaccination recommendation BAG and EKIF
Vaccination against herpes zoster with two doses and a minimum interval of 1-2 months is given in two recommendation categories [25]: A Supplemental vaccination recommendation: vaccination is recommended for all immunocompetent individuals aged ≥65 years and older, regardless of their individual history of varicella and herpes zoster. Two doses are recommended with a minimum interval of two months.
B Vaccination recommendation for two defined risk groups:
B1: Vaccination is recommended for all patients aged ≥50 years with current or future (especially cellular) immunodeficiency associated with an increased risk of herpes zoster. Two doses are recommended with a minimum interval of two months.
B2: Vaccination is recommended for patients ≥18 years of age who are currently suffering from severe immunodeficiency or who are currently or will be receiving immunosuppressive treatment in the foreseeable future. The first dose should ideally be administered ≥4 weeks before an assumed, expected, or planned onset of severe immunosuppression. The second dose with a minimum interval of one to two months after the first dose or as soon as possible at a later time during or after therapy that is favorable from a medical point of view.
SHINGRIX® is intended exclusively for intramuscular injection and only for prophylactic use and not for the treatment of confirmed clinical disease. Vaccine may be administered concurrently with non-adjuvanted seasonal influenza vaccine, 23-valent pneumococcal polysaccharide vaccine (PPV23), or diphtheria-tetanus acellular pertussis vaccine with reduced antigen content (dTpa) (different injection site). The immune response (2 doses) was not affected by prior vaccination with live attenuated HZ vaccine [1]. A minimum interval between COVID mRNA vaccination and the administration of other vaccines is not required, as with all other non-live vaccines [24].
Source: GlaxoSmithKline AG
Literature:
- Shingrix Professional Information. www.swissmedicinfo.ch, accessed October 2021.
- Federal Commission for Immunization Issues (EKIF). Evaluation of vaccination against herpes zoster according to the analysis criteria for national vaccination recommendations in Switzerland. 14 December 2015; 1-34.
- Hillebrand K, Bricout H, Schulze-Rath R, et al: Incidence of herpes zoster and its complications in Germany 2005-2009. Journal of Infection 2015 Feb; Vol 70: 178-186.
- Kawai K, Gebremeskel BG, Acosta CJ: Systematic review of incidence and complications of herpes zoster: toward a global perspective. BMJ Open 2014 Jun;4(6): e004833.
- Mahalingam R, Wellish M, Wolf W, et al: Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med 1990 Sep; 323(10): 627-631.
- Weinberg A, Lazar AA, Zerbe GO, et al: Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010; 201(7): 1024-1030.
- Kimberlin DW, Whitley RJ: Varicella-zoster vaccine for the prevention of herpes zoster. N Engl J Med. 2007 Mar;356(13): 1338-1343.
- Cunningham AL, Lal H, Kovac M, et al: Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016 Sep; 375(11): 1019-1032.
- Levin MJ: Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol 2012 Aug; 24(4): 494-500.
- Patterson-Bartlett J, Levin MJ, Lang N, et al: Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine 2007 Oct; 25(41): 7087-7093.
- Lungu O, Annunziato PW, Gershon A, et al: Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc Natl Acad Sci USA 1995 Nov; 92(24): 10980-10984.
- Furuta Y, Takasu T, Fukuda S, et al: Detection of varicella-zoster virus DNA in human geniculate ganglia by polymerase chain reaction. J Infect Dis 1992 Nov; 166(5): 1157-1159.
- Volpi A: Severe complications of herpes zoster. Herpes 2007 Sep; 14 Suppl 2: 35-39.
- Yawn BP, Wollan P, Kurland MJ, et al: Herpes Zoster Recurrences More Frequent Than Previously Reported. Mayo Clin Proc. February 2011;86(2): 88-93.
- Chlibek R, Smetana J, Pauksens K, et al: Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014 Mar; 32(15): 1745-1753.
- Bharucha T, Ming D, Breuer J: A critical appraisal of ‘Shingrix’, a novel herpes zoster subunit vaccine (HZ/Su or GSK1437173A) for varicella zoster virus. Hum Vaccin Immunother. 2017 Aug;13(8): 1789-1797.
- Lal H, Cunningham AL, Godeaux O, et al: Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015 May; 372(22): 2087-2096.
- Schwarz TF, Volpe S, Catteau G, et al: Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum Vaccin Immunother 2018 Jun; 14(6): 1370-1377.
- Merck Sharp & Dohme. Zostavax European public assessment report, Annex I: Summary of product characteristics: EMA; [updated January 2019; accessed December 2020]. Available from: www.ema.europa.eu/en/documents/product-information/zostavaxepar-product-information_en.pdf.
- Lecrenier N, Beukelaers P, Colindres R, et al: Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Review of Vaccines 2018; 17(7): 619-634.
- Dendouga N, Fochesato M, Lockman L, et al: Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine. 2012 Apr;30(20): 3126-3135.
- Leroux-Roels G, Marchant A, Levy J, et al: Impact of adjuvants on CD4(+) T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin Immunol 2016 Aug; 169: 16-27.
- US Food and Drug Administration; Vaccines and Related Biological Products Advisory Committee. Briefing document: Shingrix (Zoster Vaccine Recombinant, Adjuvanted). 2017 [accessed December 2020]. Available from: www.fda.gov/media/107553/download.
- BAG: Vaccination recommendation for mRNA vaccines against Covid-19 (as of Sept. 14, 2021), accessed at%20gegen%on Sept. 2021.
- BAG: New recommendations for vaccination against herpes zoster: vaccine SHINGRIX. FOPH Bulletin 47/2021;22: 9-15.
- Boutry C, et al: The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: Interim results of an extension study of the pivotal phase III clinical trials (ZOE-50 and ZOE-70). Clin Inf Dis 2021: 1-30.
HAUSARZT PRAXIS 2022; 17(3): 32-33