To slow the progression of chronic kidney disease, there are disease-specific and nonspecific measures. Basic diagnostics for chronic renal failure include urine status and sonography of the kidneys. If the kidney disease does not clearly match a hypertensive or diabetic nephropathy, a specialist clarification is usually useful in order to strive for an exact diagnosis and, if necessary, to enable a specific therapy. The best-evidenced nonspecific progression-inhibiting measure is treatment of proteinuric patients with ACE inhibitors or ARBs. Other measures include adequate blood pressure control, treatment of acidosis if necessary, and salt restriction. Patients with chronic kidney disease are sensitive to “second hits.” NSAIDs, phosphate-containing laxatives and iodine-containing X-ray contrast media should therefore be avoided.
The incidence and prevalence of chronic kidney disease (CKD) has steadily increased over the past decades. Etiologically, CKD can be caused by a wide variety of renal diseases. The most common – and also largely responsible for the increasing prevalence of CKD – are diabetic and hypertensive nephropathy. In addition to the risk of progressing to end-stage renal failure, CKD leads to significantly increased cardiovascular morbidity and mortality [1]. Chronic kidney disease therefore represents a relevant burden for the health care system. Most CKD patients receive primary care from primary care physicians. Therefore, it is important that patients requiring specific therapy are identified in a timely manner and that nonspecific progression-reducing measures are known and implemented.
Disease specific treatment
As mentioned, a variety of etiologically different kidney diseases can lead to CKD. Specific (e.g., immunosuppressive) therapies exist for some of these. Therefore, to enable causal therapy, a diagnosis of the underlying disease should be sought. Good triage in the primary care practice is critical to ensure that patients with specific treatable renal disease receive nephrologic evaluation. The following rules of thumb apply:
- Basic diagnostics of every patient with relevantly impaired renal function that cannot be explained prerenally and is rapidly reversible include urinalysis and sonography of the kidneys. Sonography helps to exclude a postrenal cause, may be diagnostic in cystic nephropathies, and sometimes allows inference of chronicity or potential reversibility of the nephropathy based on renal size.
- Patients with hypertensive nephropathy are usually older, show a rather slowly progressive deterioration of renal function, usually have no relevant proteinuria (at most “microalbuminuria”), an inconspicuous urine sediment, and usually a long-standing history of hypertension with other hypertensive end-organ damage. Also typical of these patients is impaired renal autoregulatory capacity with marked fluctuations in serum creatinine as a function of hydration and blood pressure. In older patients, there is often no need for a specialist examination.
- Diabetic nephropathy is typically characterized by slowly progressive albuminuria followed by a more rapid decline in glomerular filtration rate (GFR) compared with hypertensive nephropathy. By then, other microvascular complications (retinopathy) are usually found. In case of atypical course, it is worthwhile to search for alternative causes.
- In patients who do not fit into one of the above categories, especially those with relevant proteinuria, abnormal urine sediment, or rapid decline in GFR, evaluation by renal biopsy should be considered and nephrology referral is usually indicated. Here, a specifically treatable cause could underlie (e.g., ANCA vasculitis, membranous nephropathy, IgA nephropathy, etc.).
Nonspecific progression factors and their treatment
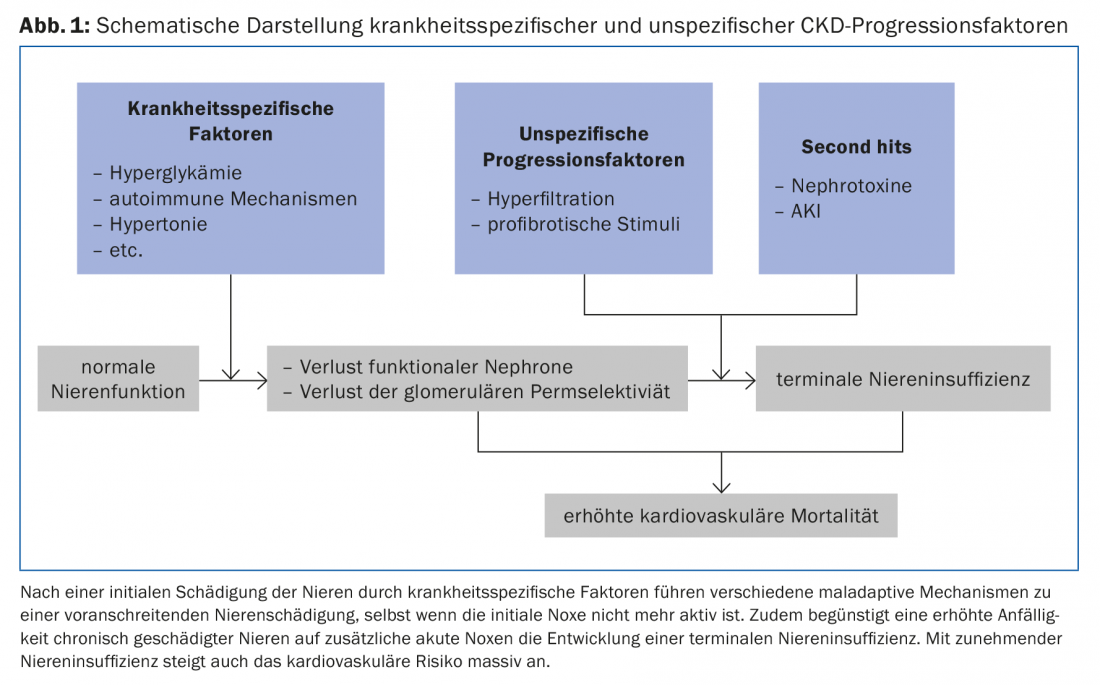
Once relevant renal damage has occurred, a number of maladaptive mechanisms contribute to further deterioration of renal function, regardless of the underlying disease (Fig. 1).
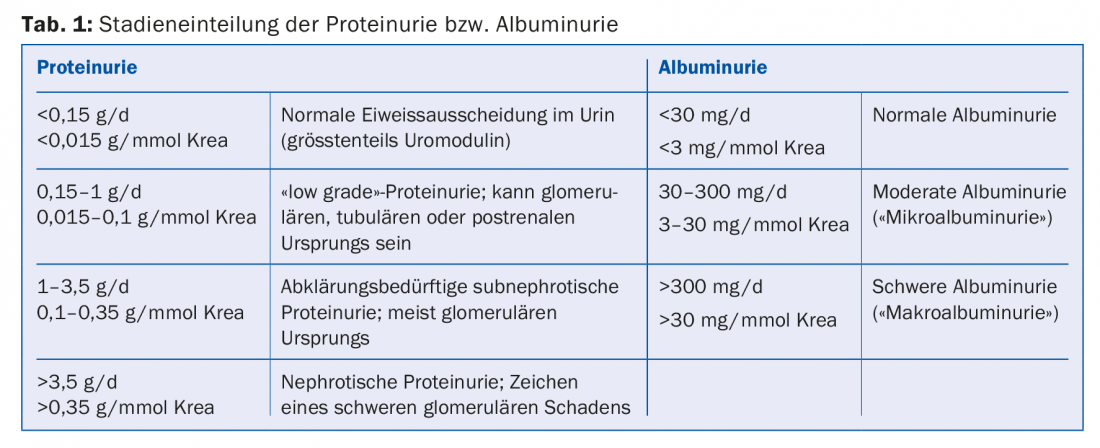
Proteinuria/albuminuria: Proteinuria or albuminuria is usually a consequence of impaired glomerular permeability (box, tab. 1). Its extent correlates with the progression of renal insufficiency [2]. Interventions that reduce proteinuria or albuminuria also slow CKD progression. ACE inhibitors and angiotensin receptor blockers (ARBs) reduce intraglomerular pressure, glomerular hyperfiltration, and albuminuria. Their efficacy in reducing progression in CKD patients with proteinuria is well established for both diabetic [3] and nondiabetic [4] patients. Dosing should be increased until proteinuria has been reduced to the target range (which varies with underlying renal disease) or the dose is limited by unacceptable side effects (e.g., hypotension/orthostatic dizziness) or the maximum allowable drug dosage has been reached. The previously advocated combination therapy of ACE inhibitors and ARBs has largely been abandoned.

Hypertension: Arterial hypertension may lead to hypertensive nephropathy, but may also promote progression of other renal diseases, and should be adequately treated. For nephroprotection, the usual target values of <140/90 mmHg seem to be sufficient in patients without pathologically elevated albuminuria, and ACE inhibitors or ARBs are not clearly superior to other drugs. In patients with severe albuminuria and probably in patients with moderate albuminuria, it is reasonable to aim for values of <130/80 mmHg. In this case, an ACE inhibitor or an ARB should be dosed out primarily, and only then should other antihypertensives be added if necessary [5]. In most patients with significantly impaired renal function, adequate blood pressure control can only be achieved with the addition of a loop diuretic. It is worth mentioning that the mentioned target values are in terms of nephroprotection and the recent SPRINT trial has shown a possible benefit of lower target values on all-cause mortality [6].
Metabolic acidosis and its correction: With increasing renal insufficiency, the acid excretion capacity of the kidneys decreases and metabolic acidosis develops in the course of time, which has negative effects on bone metabolism and musculature, among other things. Beyond these extrarenal effects, however, acid exposure also appears to damage the kidneys themselves [7]. It is known from observational studies that serum bicarbonate inversely correlates with progression in CKD patients. In the case of renal acidosis, therapy can be aimed at either reducing the acid intake via the diet (mainly contained in animal protein) or neutralizing the supplied acid by oral therapy with sodium bicarbonate. Some small randomized trials have shown an impressive progression-reducing effect for this simple and inexpensive intervention [6], but the results of larger trials are still pending. Disadvantages of bicarbonate therapy are the high “pill burden” (3-6 capsules of 500 mg NaBic are usually required for sufficient effect) and the associated sodium intake.
Diet and lifestyle interventions: Although a positive impact of physical activity and aiming for a “healthy” body weight (BMI 20-25) on the progression of renal failure has not been formally demonstrated in controlled trials, these interventions are able to favorably influence various cardiovascular risk factors as well as proteinuria.
A low protein diet has long been promoted as a measure to reduce glomerular hyperfiltration. However, the data on the efficacy of protein restriction in CKD are not entirely clear, and the existing studies predate the widespread use of ACE inhibitors and ARBs. In addition, severe protein restriction can lead to malnutrition, which has a very unfavorable prognosis if dialysis is required at a later stage. Therefore, severe protein restriction is no longer recommended today, but excessive protein intake (e.g., in the form of protein supplements for weight training) should be avoided in patients with CKD.
High salt consumption has been associated in epidemiological studies with an increase in blood pressure, an increase in proteinuria, and a more rapid decrease in GFR in renal patients [8]. Moreover, high salt intake greatly diminishes the antiproteinuric and positive prognostic effects of ACE inhibitors and ARBs [9]. Restriction of salt intake to 5-6 g NaCl/day is recommended in most patients with chronic renal insufficiency. Whether further restriction is useful or possibly even unfavorable in certain patients remains unclear at this time.
Smoking has been associated in quite a few population studies not only with cardiovascular events but also with albuminuria and renal insufficiency [10]. Few intervention studies have also been conducted for the impact of nicotine cessation on the course of CKD, but these have all been positive.
Therapeutic approaches with uncertain or no proven effect: numerous epidemiological studies show an association between hyperuricemia and renal insufficiency and cardiovascular risk, although causality is not yet fully established [11]. Single small studies have shown a beneficial effect of uricostatic therapy with allopurinol on the course of chronic renal failure. However, since allopurinol is not without risks with regard to side effects, especially in patients with impaired renal function, larger studies are needed to recommend the use of allopurinol in CKD patients without gout in principle and, if necessary, to define reasonable uric acid target levels. Febuxostat, which is not eliminated renally and has fewer side effects, could possibly play a role here in the future. Hyperlipidemia was considered as a possible progression factor of CKD based on pathophysiological considerations and epidemiological data. However, an evaluation of the SHARP trial (the largest study of lipid lowering in CKD patients) failed to demonstrate a beneficial effect of simvastatin and ezetimibe on CKD progression [12]. Thus, statins do not appear to have a relevant effect on CKD progression, but are useful for reducing cardiovascular risk in many CKD patients. Active vitamin D analogues appear to have a beneficial effect on proteinuria, but an effect on progression to renal failure remains to be demonstrated [13].
“Second hits” and their prevention: Pre-damaged kidneys are much more sensitive to acute noxae than healthy ones. This means that patients with chronic renal failure are at massively increased risk of acute kidney injury (AKI) [5], which is often incomplete recovery [14].
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most prescribed medications worldwide. In patients with severe heart failure or impaired renal function, inhibition of intrarenal prostaglandin synthesis can lead to a critical reduction in glomerular perfusion and trigger AKI [15]. NSAIDs are therefore contraindicated in patients with severely impaired renal function and should be used cautiously and for short periods in patients with mildly reduced renal function [5].
Iodine-containing X-ray contrast media may cause acute renal injury in the presence of preexisting impaired renal function, dehydration, and decompensated heart failure. The most important measures for prophylaxis of contrast nephropathy in CKD patients are: (a) Critically questioning the indication and therapeutic consequence of an examination, (b) Consider alternative examination methods depending on the problem (e.g., ultrasound, scintigraphy), (c) if an examination with X-ray contrast medium is unavoidable: prehydrate, minimize the amount of contrast medium applied and choose an isoosmolar X-ray contrast medium [5,16].
Phosphate-containing laxatives are occasionally used in colonoscopy preparation as an alternative to polyethylene glycol-containing laxatives. In the process, a considerable amount of phosphate is absorbed via the intestine. With normal kidney function, this phosphate load can be excreted relatively quickly through the kidneys. However, with impaired renal function, extremely high blood phosphate levels are reached and the consecutive very high phosphate concentration in primary urine can lead to acute calcium phosphate crystal nephropathy [17]. Phosphate-containing laxatives are therefore contraindicated with a GFR <60 ml/min/1.73m2 ; other risk factors for acute phosphate crystal nephropathy include volume depletion, diuretics, ACE inhibitors, and age [17].
Literature:
- Matsushita K, et al: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative metaanalysis. Lancet 2010; 375: 2073-2081.
- Gansevoort RT, et al: Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1): 93-104.
- Kistler AD: Albuminuria in the diabetic patient: practical management. Practice (Bern 1994). 2013;102(20): 1229-35.
- Jafar TH, et al: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001; 135(2): 73-87.
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3(1). (www.kdigo.org/home/guidelines)
- SPRINT Research Group, Wright JT Jr, et al: A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22): 2103-16.
- Kovesdy CP: Metabolic acidosis and kidney disease: does bicarbonate therapy slow the progression of CKD? Nephrol Dial Transplant. 2012;27(8): 3056-62.
- Meier P, et al: Salt and renal insufficiency. Switzerland Med Forum 2014;14(04): 50-53
- Lambers Heerspink HJ, et al: Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82(3): 330-7.
- Orth SR, et al: Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients–absence of evidence or evidence of absence? Clin J Am Soc Nephrol. 2008;3(1): 226-36.
- Jalal DI, et al: Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61(1): 134-46.
- Haynes R, et al. for the SHARP Collaborative Group: Effects of Lowering LDL Cholesterol on Progression of Kidney Disease. J Am Soc Nephrol. 2014;25(8): 1825-33.
- de Borst MH, et al: Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol. 2013;24(11): 1863-71.
- Chawla LS, et. al: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1): 58-66.
- Harirforoosh S, et al: Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009;8(6): 669-81.
- KDIGO 2012 Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012;2(1). (www.kdigo.org/home/guidelines)
- Markowitz GS, et. al: Towards the incidence of acute phosphate nephropathy. J Am Soc Nephrol. 2007;18(12): 3020-2.
HAUSARZT PRAXIS 2016; 11(12): 35-38