In the course of this year’s ASCO congress in Chicago at the beginning of June, new results of the phase Ib study on the combination of PD1 and CTLA4 inhibition were presented. They are extremely promising. In the adjuvant setting, ipilimumab alone met the primary endpoint of the recurrence-free survival.
New data from Study-004, a phase I trial that evaluated the safety, antitumor activity and pharmacokinetics of the combination of PD1 (nivolumab) and CTLA4 (ipilimumab) inhibition, offer hope for patients with advanced melanoma. Presented at this year’s ASCO Congress by Mario Sznol, MD, Yale, “Two other studies are currently underway testing this combination in later phases. Our results certainly warrant such research efforts.”
Agents were given either concurrently or sequentially (n=127). Patients were allowed to have taken up to three systemic therapies prior to participation.
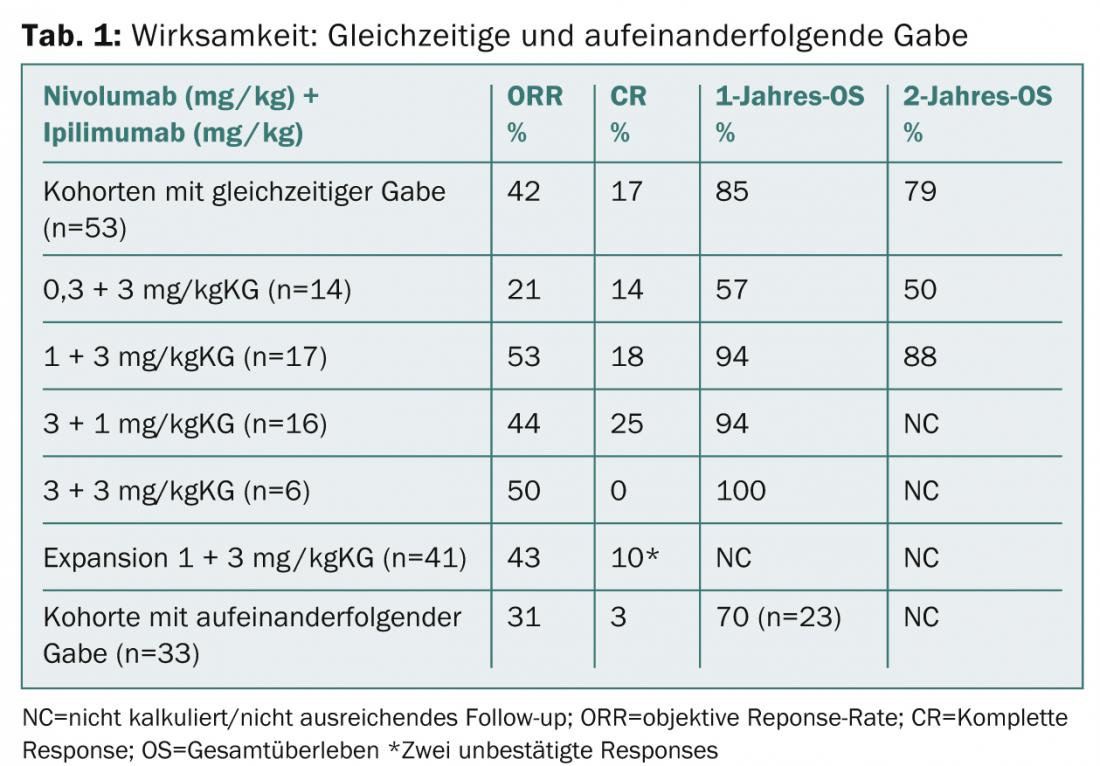
The concurrent group (nivolumab 1 mg/kgKG plus ipilimumab 3 mg/kgKG [n=17]) achieved a 1-year overall survival rate of 94, and a 2-year overall survival rate of 88%. In the extended cohort, 41 patients received the above regimen every three weeks for four doses and then nivolumab alone at 3 mg/kgKG every two weeks until progression. Research will continue at these doses in the ongoing Phase II and III trials (CheckMate-069 and -067).
Response was observed regardless of BRAF mutation status or PD-L1 release.
No new safety issues were found in the extended follow-up. Grade 3-4 therapy-associated adverse events occurred in 62% of patients in the cohorts with concomitant administration. The main results of the study update are presented in Table 1.
Ipilimumab in the adjuvant setting.
A double-blind randomized phase III trial also showed that ipilimumab at a dose of 10 mg/kgKG significantly improved recurrence-free survival (RFS, time to recurrence or death) compared with placebo, even at an earlier melanoma stage. Participants were patients with melanoma (stage III) and high risk of recurrence after complete surgical resection (adjuvant setting). This is now the third phase III trial that has been positive for ipilimumab in melanoma therapy.
“Although in stage III the likelihood of recurrence is very high. there are very few treatment options that help reduce the risk of metastasis after surgery,” said Prof. Alexander Eggermont, MD, Villejuif, who presented the results at ASCO.
The study observed a 25% reduction in the risk of recurrence or mortality (HR=0.75; 95% CI=0.64-0.90; p=0.0013). After three years, 46.5% of ipilimumab patients and 34.8% of the placebo group were relapse-free. The median RFS was 26.1 resp. 17.1 months.
Side effects were broadly comparable to those of ipilimumab in the treatment of advanced melanoma. They were mostly immune-related and well treatable within the management protocols on this agent. A higher incidence of endocrinopathies was observed. 48.8% discontinued ipilimumab therapy due to adverse events (vs. 1.7% on placebo). Five deaths were related to verum.
According to Prof. Eggermont, the results are relevant both because ipilimumab is the first immune checkpoint inhibitor to show improvement in such a treatment setting and because the benefit was observed in all subgroups, including those at highest risk of relapse. It is therefore reasonable to assume that ipilimumab could be used broadly across different lines of therapy and stages of disease in the future. Further studies investigating the compound in the adjuvant setting are currently ongoing. Meanwhile, ipilimumab at a dose of 3 mg/kgKG is approved for the treatment of advanced (non-resectable or metastatic) melanoma in adults who have received prior therapy.
Source: 50
th
Annual Meeting of the American Society of Clinical Oncology (ASCO), May 30-June 3, 2014, Chicago.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(5): 20-21.
CONGRESS SPECIAL 2014; 30-32