The combination of continuous glucose monitoring (CGM) with a sensor-controlled insulin pump has already brought us a lot closer to the vision of an “artificial pancreas”. Compared to insulin pump therapy alone, glucose fluctuations can be significantly reduced, which also has a favorable effect with regard to the development of diabetic secondary diseases, as corresponding study findings show.
Glycemic variability is an increasingly topical issue, emphasized PD Dr. med. Torben Biester, Hannover, on the occasion of the training event “Internal Medicine Interdisciplinary – Diabetology without Borders” [1]. Continuous glucose monitoring (CGM) systems are intended to help improve the controllability of insulin therapy. A reduction in the variability of glucose concentrations is associated with a reduction in the number of hypo- and hyperglycemic episodes. In this context, time in target (“TIR”) is gaining importance as a complement to HbA1c [2]. AID (automatic insulin dosing) systems – also called “hybrid closed-loop” systems – combine rtCGM with an insulin pump, while an algorithm takes over control of insulin delivery as needed. In continuous glucose monitoring, the goal is to spend as much time as possible within the established target range (TIR) of 70-180 mg /dl, the target is >70%. Less than 4% per day should be spent in the hypoglycemic range (<70 mg /dl; <3.9 mmol/l), which is captured by the Time Below Range (TbR) parameter [1]. Current findings on the risk of diabetic sequelae show that a stable glucose profile also offers longer-term benefits.
Visualization of glucose progression and optimization of therapy control
“Technology is a ‘game changer’ for visualizing metabolic status,” Dr. Biester said. Continuous measurements of blood glucose levels can capture glucose variability and hypoglycemia better than conventional blood glucose measurements. Visualization of glucose trajectories supports diabetes management by enabling easier individualized therapy management.
In CGM systems, glucose is not measured in the blood but via a small sensor in the tissue fluid of the subcutaneous fat. Continuous glucose monitoring in “real time” (rtCGM) provides near real-time glucose data. Unlike intermittent scanning glucose monitoring (iscCGM), also known as flash glucose monitoring, rtCGM can alert users when glucose levels are trending toward hypoglycemia or hyperglycemia [2]. In addition to type 1 diabetics, insulin-dependent type 2 diabetics may also benefit from CGM [3].
An example of the evaluation of insulin therapy using an analysis of CGM data is provided by a study by Bergenstal et al. [4]. In the 16-week, exploratory, parallel-group design study, 59 adults with type 1 diabetes were randomized 1:1:1 to glargine-300 or glargine-100 administered once daily in the morning or evening, with crossover in the injection schedule. It was shown that the mean 24-h glucose courses were more stable under glargine-300 regardless of whether the application was in the morning or in the evening. The rate of nocturnal hypoglycemia (<54 mg /day, SMPG) and of severe hypoglycemia proved to be significantly lower with glargine-300. Glargin-300 is one of the ultra-long-acting basal insulins.
More time on target, lower risk of secondary diseases
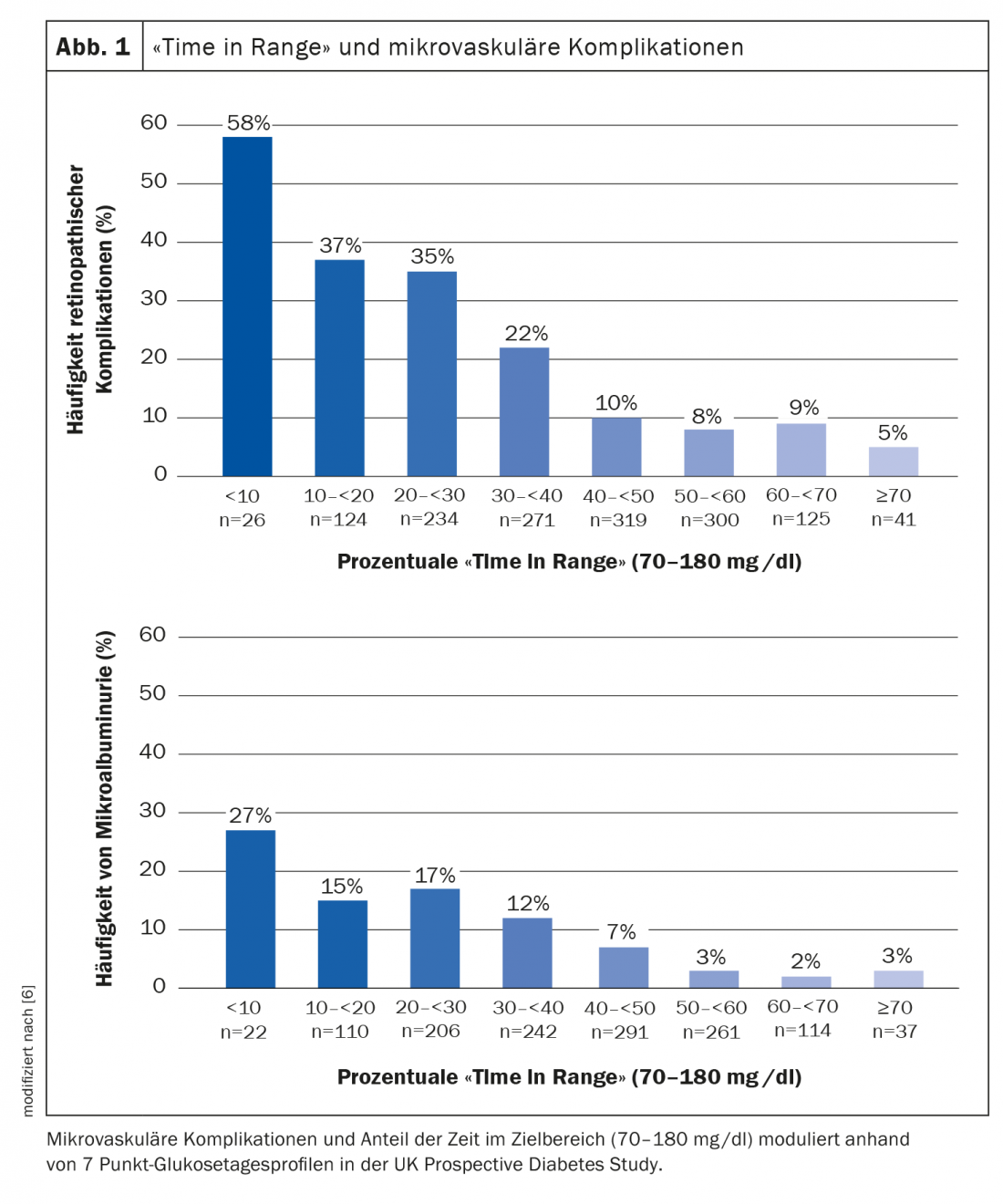
Time in Range (TIR) is a key parameter for the assessment of the current metabolic status and furthermore relevant for diabetesassociated complications [1,5]. “The ‘time in range’ seems to be a very good indicator of whether a patient has a high risk of complications,” explains Prof. Thomas Forst, MD, Mannheim [12]. High TIR levels are thought to be correlated with good long-term glucose control (=low HbA1c levels), thereby contributing to a reduction in the risk of diabetic sequelae [5]. In a study by Beck et al. a strong association between TIR and the development of microvascular complications was shown [6] (Fig. 1). The hazard rate of progression of retinopathy and development of microalbuminuria increased by 64% and 40%, respectively, for each 10% lower TIR [5,7].
AID: individualized insulin therapy
The basic principle of “Hybrid-Closed-Loop” resp. automatic insulin dosing (AID) is that the amount of insulin is automatically adjusted to the glucose data. This not only allows control of the basal rate, but corrections are also made automatically to achieve a predetermined target value. The function of a healthy pancreas is thus virtually mimicked, although there are still some differences [1]. The goal of completely automated insulin dosing has not yet been achieved. The systems still require input on meals and planned physical activity. However, the algorithms learn themselves and make individualized bolus suggestions, for example. Studies and empirical data show that the use of AID systems leads to a reduction in the variability of glucose concentrations, which is associated with an improvement in “time in range” and a reduction in the number of hypo- and hyperglycemic episodes [8]. The use of AID systems will become more important in the future. One goal is to ease the burden on diabetics during their therapy. When selecting the appropriate system, attention should be paid to individual conditions and requirements. Interoperability of diabetes technologies – especially the linking of sensor and insulin pump – is a big topic. In addition to high measurement accuracy, intelligent algorithms are needed for communication between the devices, since the transmitted glucose values form the basis for calculating the insulin dose. For proper application, it is important to understand the technique and algorithm of AID systems [3]. Therefore, comprehensive training on technical aspects, data analysis and customization is a prerequisite for successful use. Ideally, patients can lead a largely normal life without fear of acute metabolic derailments.
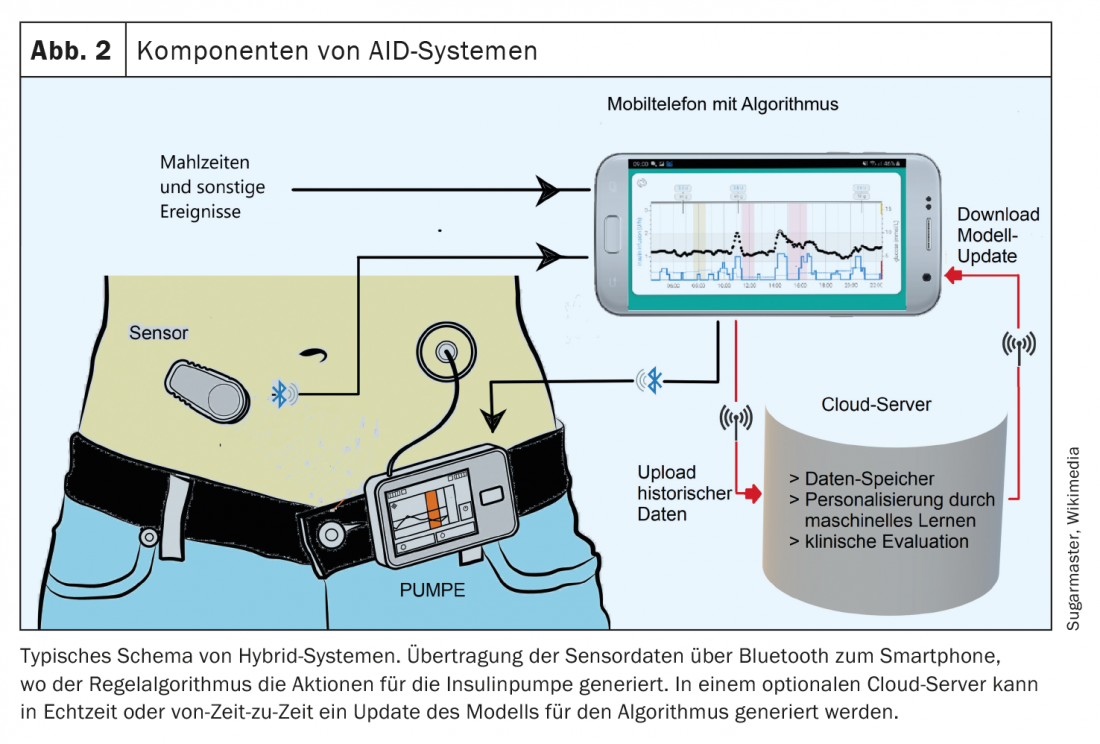
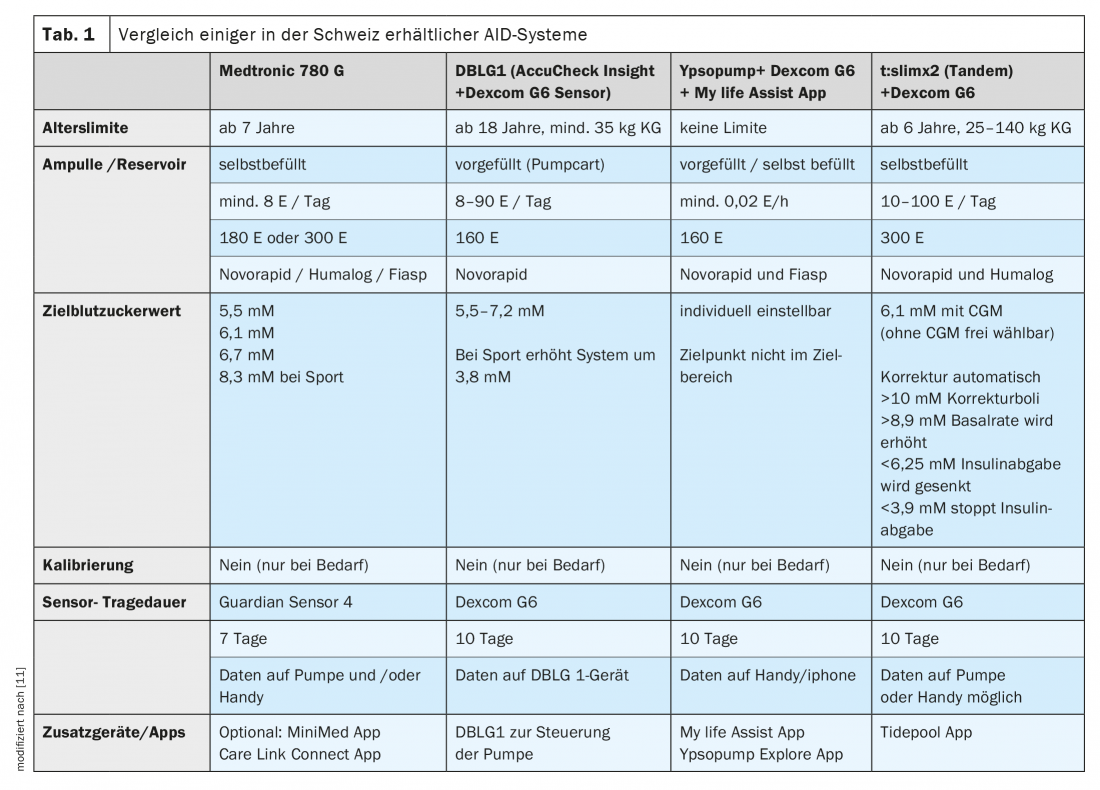
Two of the newest additions to the Swiss market include the following hybrid closed-loop or AID systems:
- t:slim X2 [9]: This glucose sensor-coupled insulin pump system uses Control-IQ software. The pump is very compact and has a modern touch screen. Coupling is done with the Dexcom G6 sensor, directly with the pump, so no additional device is needed. Meal times and amount of carbohydrates consumed in each case must be entered. This also applies to the carbohydrate factors, i.e. the dose of insulin required per amount of carbohydrate.
- DBLG1 algorithm [10]: The CE-certified DBLG1 algorithm connects the Accu Chek Insight pump and the Dexcom G6 sensor via a smartphone-like device. With this system, the amount of carbohydrate consumed must be entered, but not the carbohydrate factors. This is because the software that connects the Dexcom sensor to the Insight pump automatically controls insulin delivery. It is therefore a self-learning system that adapts to the individual user.
Literature:
- Biester T: TIR and glycemic variability: 2nd generation basal insulins beneficial? PD Torben Biester, MD. Diabetology without borders, 04.02.2022.
- Danne T, et al: Time in Range: A new parameter – complementary to HbA1c. Dtsch Arztebl 2019; 116(43).
- Kröger J, Kulzer B: New forms of glucose monitoring and the impact on therapy and training in Germany. Diabetes Health Report 2021, 173-182.
- Bergenstal RM, et al: Diabetes Care 2017; 40(4): 554-560.
- DDG/AGDT: Time in Range 2021 statement, www.deutsche-diabetes-gesellschaft.de (last accessed Mar. 14, 2022).
- Beck RW, et al: Diabetes Care 2019; 42(3): 400-405.
- Lu J, et al: Diabetes Care. 2018;41(11): 2370-2376.
- German Diabetes Aid: AID System, www.diabetesde.org (last accessed Mar. 14, 2022).
- Metabolism Center St.Gallen, www.friendlydocs.ch/2021/11/02/neue-sensor-gekoppelte-insulinpumpe-tslim-x2 (last retrieved 03/14/2022)
- St. Gallen Metabolic Center, www.friendlydocs.ch/2021/05/17/neu-roche-insulinpumpe-jetzt-sensor-gekoppelt (last accessed 03/14/2022).
- Kantonsspital Aarau: www.ksa.ch/sites/default/files/cms/edm/pocketguide/appendix/13_hybrid-closed-loop_systeme.pdf (last accessed 14.03.2022)
- Forst T: From tissue glucose sensor to sensor-controlled insulin pump. Prof. Dr. med. Thomas Forst, Diabetology without borders, 04.02.2022.
HAUSARZT PRAXIS 2022, 17(4): 20-22