Immune checkpoint inhibitors are finding increasingly widespread use in oncology with some breakthrough success. However, the activation of the immune system also triggers a wide range of side effects. Neurological side effects are also observed – rare, but then potentially serious. Then good management is required.
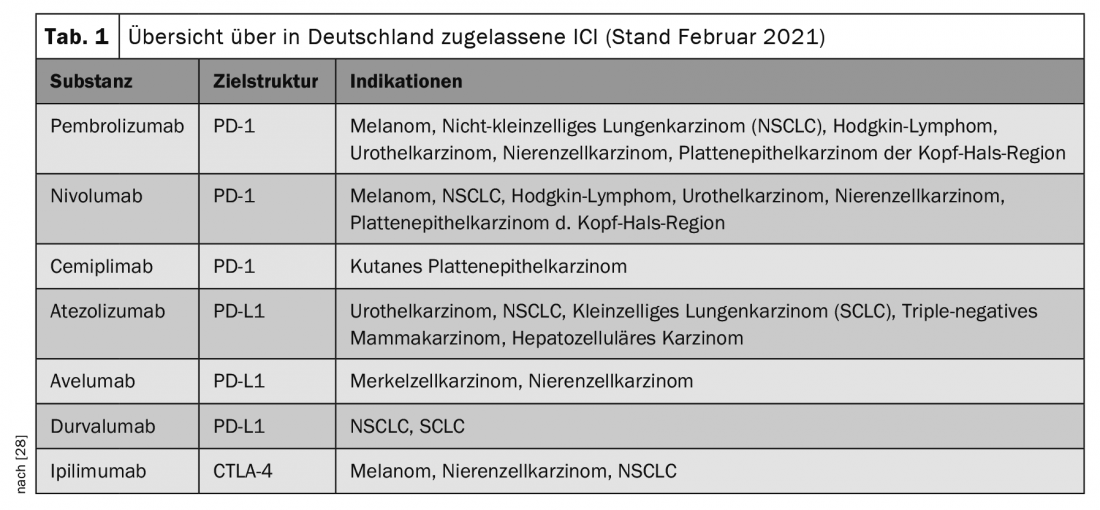
In oncological therapy, so-called immune checkpoint inhibitors (ICI) have been used more and more in recent years and have led to a breakthrough in the treatment of various malignancies, sometimes with long-lasting or permanent remissions. The indications are constantly being expanded and new substances are being approved, so that ICIs are being used in the treatment of an increasing number of tumor types (Table 1).
ICI are specific antibodies directed against immune checkpoints. Examples of such checkpoint molecules are programmed cell death 1 receptor (PD-1), PD-1 ligand (PD-L1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4) [1].
Under physiological conditions, immune checkpoints in T and B cell maturation occupy a key site for self-tolerance and modulation of the immune system. Thus, CTLA-4 has mainly an inhibitory function in T cell priming, while PD-1 and PD-L1 as a receptor/ligand pair play an inhibitory role in the effector phase, i.e., in the attack of the T cell with already specific T cell receptor on the tumor cell [2]. Tumor cells use these checkpoint molecules for immune invasion by activating them to inhibit the body’s own immune system directed against them. For example, many tumor cells express PD-L1 and can use this mechanism to inhibit specific T cells directed against the tumor cells by binding to their PD-1 receptor and escape destruction by the body’s own immune system. This immune evasion should be prevented by blocking the above receptors with ICI, thus promoting the anti-tumor immune response.
Research is ongoing on combinations of conventional chemotherapy, tyrosine kinase inhibitors, radiotherapy and ICI, as well as on other checkpoints as targets or on the combination of different ICIs. Studies found evidence of a synergistic effect of local radiotherapy followed by systemic ICI administration. It is believed that tumor tissue disintegrated by radiotherapy with released antigens and tumor-specific neoantigens may promote T-cell priming, resulting in a stronger anti-tumor response systemically [2].
However, due to ICI-mediated activation of the immune system, it is not surprising that a wide spectrum of autoimmune phenomena (immune-related adverse event; irAE) can be triggered or existing autoimmune and paraneoplastic diseases can be triggered as side effects. Depending on the checkpoint inhibited, irAEs were observed in 70-90%, of which most were of mild severity (grade 1 or 2 according to CTCAE (Common Terminology Criteria for Adverse Events)) [1]. The organs most commonly affected by irAE are the intestine, skin, lungs, liver and endocrine organs. In contrast, neurological irAEs (nirAEs), which can affect the central and peripheral nervous systems, neuromuscular endplate, and musculature, are rare [3]. With the growing use of ICIs, knowledge about nirAE is continuously increasing, although most evidence to date has been generated primarily through case reports, retrospective case series, and meta-analyses; large prospective adverse event studies have been lacking. Therefore, the reported frequencies of nirAE differ greatly. Grade 1-2 nirAEs were observed in 6-12%, and severe grade 3-4 nirAEs in 0.1-1% of treated patients [4]. Pre-existing autoimmune disease does not appear to confer a significantly increased risk of developing new irAEs with ICI therapy [5]. However, this is controversially discussed due to the still very limited data situation. On average, irAEs under ICI therapy occur within the first 12 weeks after initiation of ICI therapy [6]. In individual cases, however, the duration may vary widely, from a few days after the first ICI administration to more than a year after the start of therapy or even after ICI administration has ceased.
Of course, other neurologic differential diagnoses must be ruled out by appropriate diagnostic means. Potential nirAEs should be identified and treated early because, although rare, some are associated with high morbidity and mortality [7,8].
Here, an overview of the currently known nirAE will be given. The ICI-associated diseases partly mimic well-known neurological syndromes, but occur in unusual combinations or represent a new phenotype. However, the severity, therapeutic management, and course differ significantly from classic neurological conditions.
Peripheral nervous system
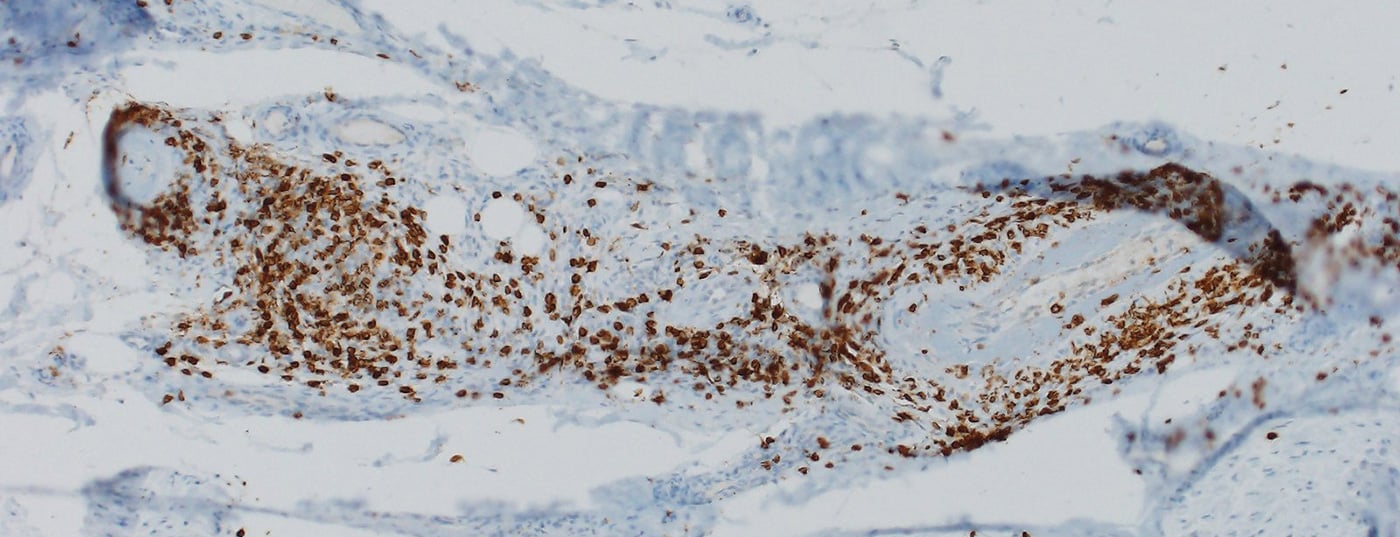
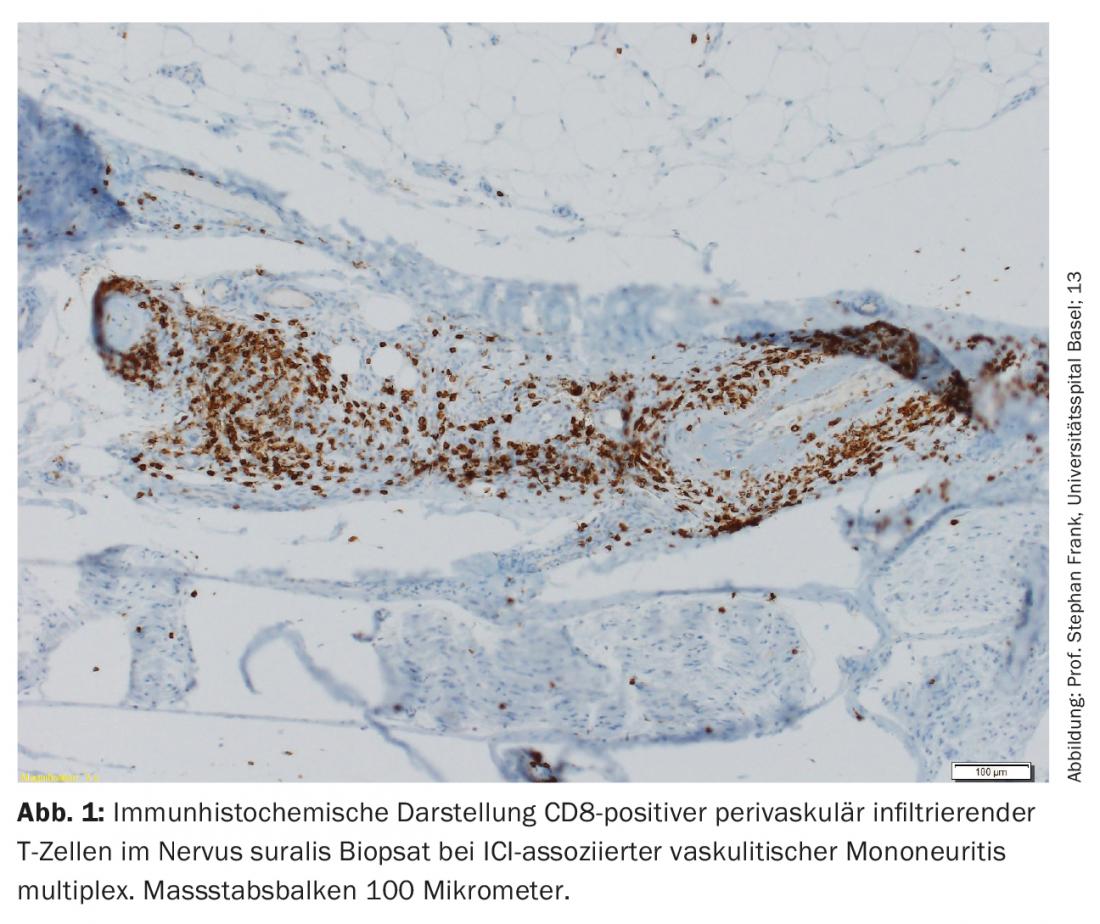
The majority of nirAEs involve the peripheral nervous system [9]. Polyneuropathies were observed in approximately 3% of patients treated with ICI. ICI-associated neuropathies can be both axonal and demyelinating and have different patterns of distribution, including mononeuropathy, mononeuritis multiplex, symmetric polyneuropathy, small fiber neuropathy, autonomic neuropathy, neuralgic amyotrophy, meningoradiculitis, and cranial nerve involvement [10,11]. Vasculitic peripheral polyneuropathies and p-ANCA-positive mononeuritis multiplex have also been described (Fig. 1) [12,13]. Depending on the severity, pausing ICI, steroid administration, and/or plasma separation (to remove antibodies that are still therapeutically effective) must be considered. However, often only mild symptoms exist, so continuation of ICI therapy can be done depending on the overall context [9,10]. The response of ICI-associated neuropathies to steroids is good.
Paradigmatic is an acute inflammatory demyelinating polyneuropathy (AIDP), in which the classical picture of a cytalbuminous dissociation with high protein and low cell count is less found in the CSF, but a mild pleocytosis (up to 15 cells/μl) is characteristic in addition to the increased protein [9]. A chronic course (CIDP) is rare. In the case of AIDP (Guillain-Barré-like syndrome), ICI administration should be permanently stopped in view of the potential threat to life, and high-dose steroid administration in combination with intravenous immunoglobulins (IVIG) or plasmapheresis should be started [14].
Painful myositis with truncal paresis as the main symptom has also been described, especially under anti-PD-1 therapies [15]. They sometimes occur in isolation, but often in combination with myasthenic syndrome or AIDP-like disease. Concomitant myocarditis is common and should always be considered because it has a high mortality (approximately 1/3 of cases) [16]. Dermatomyositis, polymyositis as well as acute necrotizing myositis with a characteristic histopathological phenotype are known [9]. Specific paraneoplastic or autoimmune antibodies have not been detected to date. In most cases, symptom improvement occurred after stopping ICI administration and immunosuppression.
Myasthenia gravis, in 60% with positive anti-AChR antibodies, was diagnosed in approximately 0.1-0.2% of patients receiving ICI therapy [17]. Mostly ocular and bulbar regions were predominantly affected, followed by proximal extremities. In a large proportion, creatine kinase (CK) was concomitantly elevated as a sign of concomitant (cardio)myositis [15]. The course was often severe with high mortality. Accordingly, permanent discontinuation of ICI and immunosuppressive therapy is recommended. In contrast to classic myasthenia gravis, the (well-monitored) early use of high-dose steroids is also recommended here because of the good response [14]. Because steroids can lead to a transient exacerbation of muscle weakness with the risk of respiratory failure, consideration should be given to administering IVIG or performing plasmapheresis before steroids [3]. The response to symptomatic therapy with e.g. pyridostigmine varies.
Central nervous system
Associated encephalitides were observed in 0.1-0.2% of patients treated with ICI. Paraneoplastic antibodies such as anti-Ma-2, anti-Hu, anti-CASPR2, or anti-NMDA receptor antibodies were frequently found [18,19]. Pathologic CSF findings with lymphocytic pleocytosis are typical, MRI findings vary from unremarkable to T2 hyperintensities to isolated cases with regional contrast uptake in the neurocranium [20]. The course is usually favorable when ICI administration is stopped and steroids are used, although fatal courses have been reported [21,22].
Endocrinologic disorders are diagnosed in 4.9-17% in the setting of hypophysitis, especially during therapy with the anti-CTLA-4 antibody ipililumab at doses greater than 3 mg/kg. At lower doses, the incidence is significantly nierdriger [23]. Because the immune response quickly damages the pituitary irreversibly, long-term hormone replacement is usually necessary. Accordingly, the current therapy recommendation is to substitute the appropriate hormones, to pass on the ICI and to administer steroids only in individual cases, as this rarely leads to a relevant improvement [24].
Especially under CTLA-4 inhibition, aseptic meningitis was observed in 0.1-0.2% of patients. In the CSF, in addition to the increased cell count in the absence of pathogen detection, a protein increase was often found. With steroid therapy and discontinuation of ICI administration, the symptoms were mostly regressive [3].
ICI-associated exacerbations of preexisting inflammatory CNS diseases such as multiple sclerosis have been described in isolated cases, as has de novo development of multiple sclerosis. However, therapy can be performed under close neurologic monitoring in multiple sclerosis [25,26]. Aquaporin-4 antibody-mediated neuromyelitis optica and seronegative transverse myelitis have also been reported. Also described have been isolated cases of ICI-associated tolosa-hunt syndrome, neurosarcoidosis, PRES-like syndromes, or CNS vasculitis [27,28].
Summary therapy recommendation
Therapy recommendations are based on expert experience and case reports due to lack of prospective data. As a general rule, ICI therapy should be paused immediately in cases of severe irAE. For milder irAE (grades 1-2), ICI administration can often be resumed. These are usually case-by-case decisions, weighing the severity of the nirAE and the risk of tumor progression if ICI administration is stopped. In non-neurologic irAE, for which more data are available because of the higher incidence, the response to steroids is usually good. This also applies to nirAE. The extent to which combination with immunoglobulins and/or plasma separation is appropriate depends on the severity of the nirAE and the type of ICI (e.g., monotherapy versus combination therapy). Remarkably, ICI-associated AIDPs often respond well to steroids, in contrast to classic Guillain-Barré syndrome. Due to the long ICI half-life of 2-4 weeks depending on the preparation, it is recommended to administer prednisolone at 0.5-2 mg/kgKG, depending on the severity of the nirAE, for 2-4 weeks and then slowly taper the steroid dose. Recurrences of irAE on steroid reduction are common, so they should be reduced only slowly over a few weeks [24]. If symptomatology is not steroid sensitive, intravenous immunoglobulins (IVIG) or plasmapheresis may be used [3]. Prospective studies are needed to optimize treatment regimens and to select patients for different treatment options (monotherapy versus combination therapy). An essential contribution to the therapeutic safety of these often very complex and multimorbid patients is the interdisciplinary cooperation of neurologists and oncologists. Specific contact persons should be defined here.
Take-Home Messages
- nirAEs as rare but potentially serious complications of ICI therapy.
- Any type of autoimmune disease possible as nirAE, more often affecting the peripheral nervous system.
- Depending on severity, therapeutic discontinuation of ICI and/or steroids as primary therapy.
Literature:
- Heinzerling L, De Toni E, Schett G, et al. (2019): Checkpoint inhibitors. Dtsch. Arztebl. Int. 116: 119-126
- Riggenbach E, Ermiş E, Elicin O, et al. (2021): Combination of radio- and immunotherapy. Swiss Med Forum 21(5-6): 78-82.
- Astaras C, de Micheli R, Moura B, et al. (2018): Neurological Adverse Events Associated with Immune Checkpoint Inhibitors: Diagnosis and Management. Curr. Neurol. Neurosci. Rep. 18
- Martins F, Sofiya L, Sykiotis GP, et al. (2019): Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16: 563-580
- Menzies AM, Johnson DB, Ramanujam S, et al. (2017): Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28(2): 368-376.
- Weber JS, Hodi FS, Wolchok JD, et al. (2017): Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35(7): 785-792.
- Hottinger AF (2016): Neurologic complications of immune checkpoint inhibitors. Curr. Opin. Neurol. 29: 806-812
- Kao JC, Liao B, Markovic SN, et al. (2017): Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol 74(10): 1216-1222.
- Harrison RA, Tummala S, de Groot J (2020) Neurologic toxicities of cancer immunotherapies: a review. Curr. Neurol. Neurosci. Rep. 20
- Gu Y, Menzies AM, Long GV, et al (2017): Immune mediated neuropathy following checkpoint immunotherapy. J. Clin. Neurosci. 45: 14-17
- McNeill CJ, Fehmi J, Gladwin J, Price C (2019): A rare case of Miller Fisher variant of Guillain-Barr é Syndrome (GBS) induced by a checkpoint inhibitor. BMJ Case Rep 12(8).
- Daxini A, Cronin K, Sreih AG: Vasculitis associated with immune checkpoint inhibitors – a systematic review. Clinical Rheumatology 2018; 37: 2579-2584.
- Baldauf MC, Kapauer M, Joerger et al. (in press, accepted for publication February 2021): Pembrolizumab-associated CD8+ vasculitic mononeuritis multiplex in a patient with mesothelioma. Neurology: Neuroimmunology & Neuroinflammation.
- Thompson JA, Schneider BJ, Brahmer J, et al. (2020): Management of immunotherapy-related toxicities, version 1.2020 featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw 18(3): 231-241.
- Touat M, Maisonobe T, Knauss S, et al. (2018): Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 91(10): e985-e994.
- Moreira A, Loquai C, Pföhler C, et al. (2019): Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 106:12-23.
- Makarious D, Horwood K, Coward JIG (2017): Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur J Cancer 82: 128-136
- Mongay-Ochoa N, Vogrig A, Muñiz-Castrillo S, Honnorat J (2020): Anti-Hu-associated paraneoplastic syndromes triggered by immune-checkpoint inhibitor treatment. J Neurol 267: 2154-2156
- Nowosielski M, Di Pauli F, Iglseder S, et al. (2020): Encephalomyeloneuritis and arthritis after treatment with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflammation 7(4).
- Nishijima H, Suzuki C, Kon T, et al. (2021): Bilateral Thalamic Lesions Associated With Atezolizumab-Induced Autoimmune Encephalitis. Neurology 96(3): 126-127.
- Touat M, Talmasov D, Ricard D, Psimaras D (2017): Neurological toxicities associated with immune-checkpoint inhibitors. Curr. Opin. Neurol. 30: 659-668
- Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, et al. (2019): A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med 25(8): 1243-1250.
- Maker AV, Yang JC, Sherry RM, et al. (2006): Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother 29(4):455-463.
- Haanen JBAG, Carbonnel F, Robert C, et al. (2017): Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4): iv119-iv142.
- Donia M, Pedersen M, Svane IM (2017): Cancer immunotherapy in patients with preexisting autoimmune disorders. Semin. Immunopathol. 39: 333-337.
- Hasan Ali O, Berner F, Ackermann CJ, et al. (2020): Fingolimod and tumor-infiltrating lymphocytes in checkpoint-inhibitor treated cancer patients. Cancer Immunol Immunother 70(2).
- Narumi Y, Yoshida R, Minami Y, et al. (2018): Neuromyelitis optica spectrum disorder secondary to treatment with anti-PD-1 antibody nivolumab: The first report. BMC Cancer 18(1).
- Fan S, Ren H, Zhao L, et al. (2020): Neurological immune-related adverse events associated with immune checkpoint inhibitors: A review of the literature. Asia Pac J Clin Oncol. 16: 291-298.
- Rote Liste® Service GmbH Red List. www.rote-liste.de. Accessed 27 Feb 2021
InFo NEUROLOGY & PSYCHIATRY 2021; 19(2): 6-9.