Today, the treatment of lung cancer is much more differentiated and also more complicated than it was ten years ago. This is due not least to the great dynamism in the development of new therapeutic options; in immunotherapy in particular, there are many new drug developments that can be used in a targeted manner. With the revision of the guideline on lung cancer, the current standard of therapy has now been mapped.
About 4700 people develop lung cancer in Switzerland every year [1]. Only ten to 20 percent of those affected survive the subsequent five years. This makes lung cancer the cancer with the highest mortality rate among all tumor diseases. In order to significantly improve prevention, diagnosis, therapy, and follow-up, the new S3 guideline on lung cancer was published in November 2022 after three years of work during the German Cancer Congress in Berlin [2]. With the new findings, it will be possible to improve treatments and increase patients’ chances of survival, he said. Physicians would also gain a better basis for making decisions about care through evidence-based and formally consented recommendations, explained Professor Torsten Bauer, President of the German Society of Pneumology and Respiratory Medicine (DGP) [3].
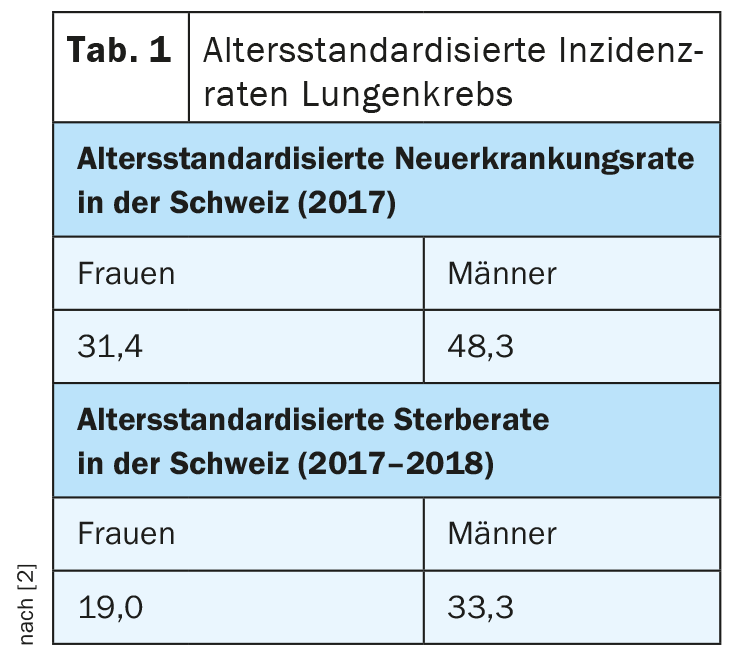
As in Germany, lung cancer in Switzerland is the second most common tumor in men after prostate cancer and the third most common after breast and colon cancer in women. Overall, it accounts for 11% to 12% of all cancer diagnoses in both countries. In the 5-year period 2014-2018, 10,024 men and 6302 women died from lung cancer. Thus, lung cancer remains by far the most common cause of cancer death among men and the second most common among women (Table 1) .
Taken into account the progress of recent years
The guideline, first published in 2010, was last updated in 2018. “Our goal with this second update is to better support physicians, affected patients, and citizens at increased risk for lung cancer in making medical decisions. In addition, with the updated version we have created a basis for targeted medical education, training and continuing education measures,” says Prof. Dr. Wolfgang Schütte, overall coordinator of the guideline and one of the lead authors. Because of the variety of treatment options that have developed in recent years, there is increasing individualization in the treatment of lung cancer, the guideline authors write. Accordingly, the expansion of the therapeutic spectrum has led to numerous modifications and innovations in the guideline. New recommendations added to the guideline include:
Computed tomography (CT) of the thorax should be used in asymptomatic individuals at risk for lung cancer. Increased risk is defined here by age between 50 and 75 years or less, smoking history of ≥15 cigarettes per day for at least 25 years or ≥10 cigarettes per day for at least 20 years, and absence or less than 10 years of nicotine abstinence.
Outside of risk-adapted screening, a validated online probability calculation model (e.g., Mayo Clinic, Herder, Brock) should be used to estimate the malignancy of a new-onset pulmonary nodule in case of incidental findings.
CT follow-up should be omitted in patients without malignant (pre-existing) disease and at low risk for lung carcinoma with a pulmonary nodule <5 mm (or <80 mm³) or in patients whose general condition does not allow further evaluation or therapy.
Non-small cell lung cancer (NSCLC) should be molecularly tested for EGFR mutations regardless of subtype early stages as well as in recurrence.
Unless contraindications exist, chemo-immunotherapy with platinum/etoposide and a PD-L1 antibody (atezolizumab or durvalumab) should be offered to patients with distant metastatic small cell lung cancer in the first line. For patients with brain metastases, the addition of a PD-L1 antibody to chemotherapy may be offered.
Every patient with newly diagnosed lung cancer should present to a thoracic oncology tumor board, where decisions are based on current guidelines. However, a deviating therapy decision could also be made under certain conditions.
Further revisions are planned as early as 2023: Due to the dynamics and ongoing developments in this field of research, it is planned to continue the lung cancer guideline as a so-called living guideline.
Literature:
- www.bfs.admin.ch/bfs/de/home/statistiken/kataloge-datenbanken/tabellen.assetdetail.20744807.html; last accessed Nov. 22, 2022.
- S3 guideline Prevention, diagnosis, treatment, and follow-up of lung cancer (version 2.0, 2022); www.leitlinienprogramm-onkologie.de/leitlinien/lungenkarzinom; last accessed 11/22/2022.
- Press release German Society for Pneumology and Respiratory Medicine e.V., 14.11.2022.
InFo PNEUMOLOGY & ALLERGOLOGY 2022; 4(4): 26.