According to the guidelines, omalizumab can be used if therapy with dosed H1 antihistamines does not lead to sufficient relief of symptoms. There is ample evidence of efficacy for this anti-IgE antibody with respect to urticaria. Findings in the autologous serum test and basophil histamine release assay have been shown to be relevant for individual differences in treatment response.
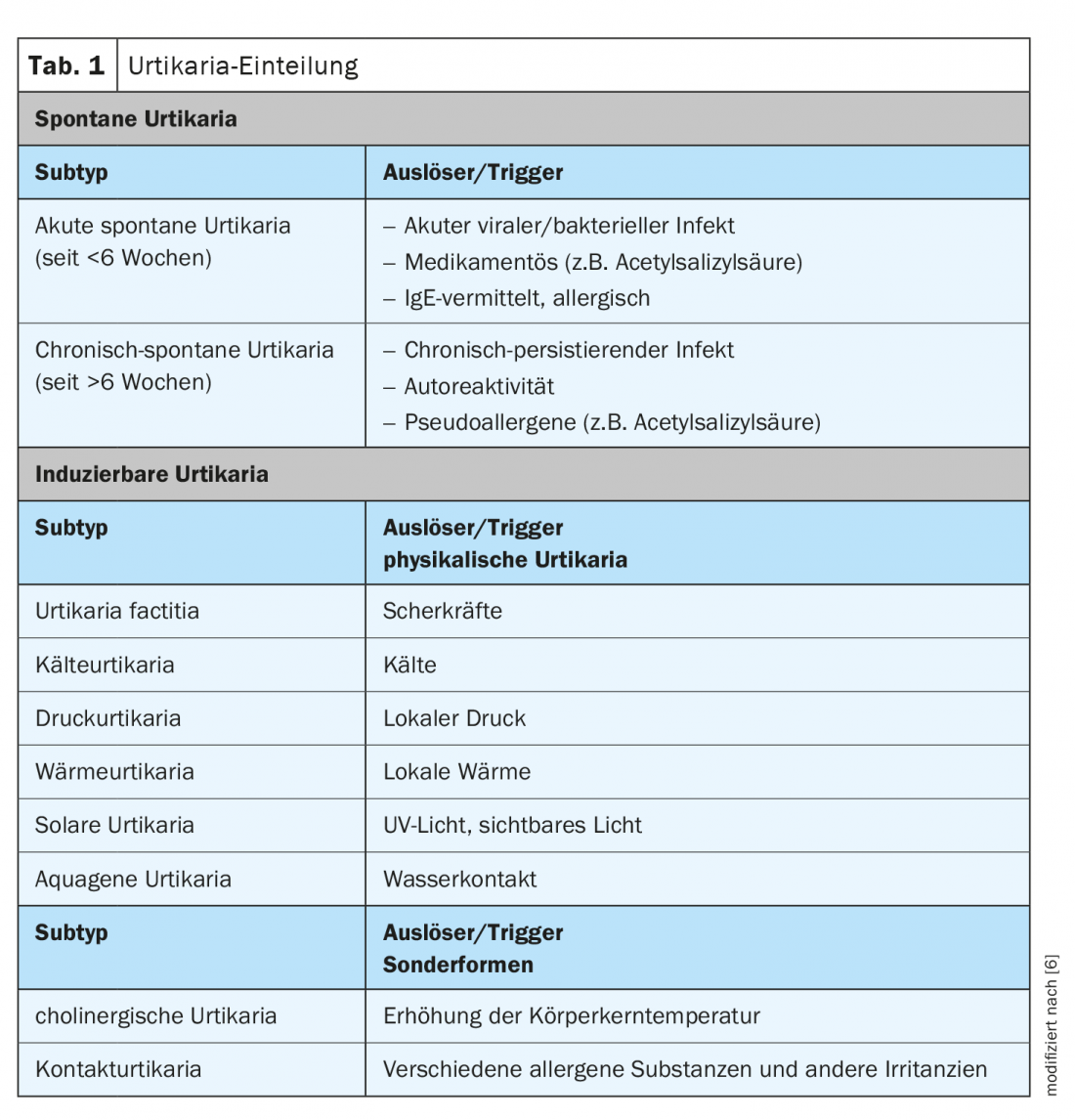
Urticaria is a common mast cell-mediated disease with a lifetime prevalence of approximately 20% [1]. Typical symptoms are wheals, angioedema, or both. If the symptoms persist for more than 6 weeks, it is chronic urticaria according to the current classification, otherwise it is acute urticaria. In chronic urticaria, a distinction is made between an inducible and a spontaneously occurring form (Tab. 1) . It is a burdensome health restriction that affects various areas of life. As part of the virtual dermatology and allergology refresher, Dr. med. univ. Sabine Altrichter, Charité Universitätsmedizin Berlin (D) on current findings in this field [2]. The EAACI/GA²LEN/EDF/WAO guideline highlights evidence-based diagnostic and therapeutic approaches for various forms of urticaria. The overall goal of treatment is to achieve freedom from symptoms. In chronic spontaneous urticaria, the focus is on drug therapy; in the chronic inducible form, avoidance of trigger factors may also provide relief [2].
Omalizumab as adjuvant therapy for chronic spontaneous progression
As first-line treatment, therapy with the following non-sedating H1 antihistamines is recommended: Loratadine, desloratadine, cetirizine, levocetirizine (up-dose to a maximum of 2-0-2). If the response is good, there is nothing wrong with continuing the therapy for a longer period of time, the speaker said. In case of side effects or insufficient response, other antihistamines may be used. If persistent chronic spontaneous urticaria cannot be adequately controlled with antihistamines and no underlying cause for the urticaria could be found, omalizumab (Xolair®) is recommended [3]. Xolair® is approved for this indication in Switzerland for adults and children 12 years of age and older. Omalizumab is a monoclonal antibody against IgE, which is to be applied subcutaneously. The recommended dosage is 2×150 mg at 4-week intervals.
The EAACI/GA²LEN/EDF/WAO guideline states the following instructions for the use of omalizumab: Before starting therapy, blood pressure should be measured and a blood count should be done (CAVE: hypertension, renal function impairment). The dosage recommendation is 2 mg/kg bw – max 5 mg/kg bw). The suggested duration of treatment is 6-12 months; if necessary, combination therapy with low-dose ciclosporin A should be considered. Administration of the first three doses should be done in the physician’s office with a subsequent 30-minute follow-up period. Further injections can be performed by the patient at home. The fear of an allergic drug intolerance reaction, as has sometimes occurred in asthma patients, has so far not been confirmed in urticaria patients, explains Dr. Altrichter. To assess the efficacy of omalizumab, he said, it is useful to administer at least three injections; response to treatment varies individually.
Therapy response correlates with basophil histamine release assay findings
In a large proportion of urticaria patients, there is a significant reduction in symptoms after the first injection, within a short time, but in some patients it takes several weeks before therapy effects are seen. The differences in the response to therapy seem to depend on whether the urticaria is autoreactive, i.e. whether the mast cells responsible for the formation of wheals are activated by the body’s own substances. The autologous serum test is used for detection; a positive finding applies to about one third of all urticaria patients. Activation of mast cells and basophils can be detected by the basophil histamine release assay (BHRA) if the autologous serum test is positive. In a study conducted at Charité Universitätsmedizin Berlin, a positive BHRA finding was shown to be a predictor of delayed response to omalizumab [4]. The mean age of the study participants was 47 years (range, 23-85 years), and the mean duration of chronic spontaneous urticaria was 3 years. Symptoms could not be adequately controlled by up-dose antihistamine treatment. Omalizumab 300 mg was administered at 4-week intervals (three injections in total). The efficacy of therapy was operationalized by measuring the Urticaria Activity Score [7], where a score ≤6 was assessed as a response. After a 12-week period, the response rate was 88% (n=56), and the proportion of non-responders was 12% (n=8). Of the 56 responders, 70% (n=39) showed a response within 8 days (“fast responders”). In 30% (n=17), it took 8 days to 3 months to achieve a response to therapy (“slow responders”).
From the observation that BHRA correlates with duration to response, the authors deduced that in these patients omalizumab results in a reduction in FcεRI expression [4]. To this end, Metz et al. conducted a study in which they investigated the effects of omalizumab on the concentration of high-affinity receptor-positive (FcεRI+) and IgE-positive (IgE+) dermal cells and basophils in blood. This demonstrated that omalizumab treatment was associated with a reduction in FcεRI+ and IgE+ basophils and intradermal cells [5].
How to further treat in the maintenance phase?
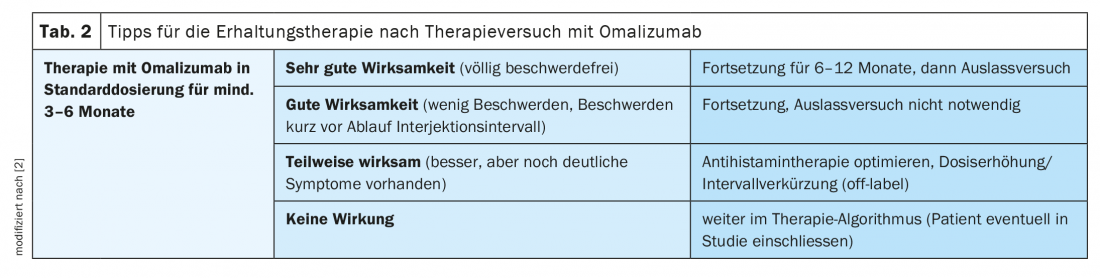
In the current version of the guideline, there is no recommendation on how to proceed after a temporary therapy with omalizumab, the speaker explained, and gave some advice based on experience (Tab.2). In patients who achieved complete freedom from symptoms, therapy should be continued for at least 6 months up to 1 year. After that, one should make an outlet attempt, as urticaria can also be self-limiting. In patients with good efficacy who report increasing symptoms shortly before the end of the injection interval, an attempt to discontinue the treatment would not be effective due to persistent disease activity. In cases where efficacy is observed but treatment results are not sufficient, an optimized antihistamine treatment could be tried or, at best, a higher omalizumab dose or a shorter injection interval (off-label). If this does not bring the hoped-for effect, Dr. Altrichter recommends continuing according to the therapy algorithm. “This is especially useful when patients have low total IgE and a positive finding on BHRA.” In a next step, ciclosporin A can be prescribed; the therapy largely corresponds to that for atopic dermatitis. The duration of therapy is 6-12 months. Important: check hypertension and renal function beforehand. There is also the possibility of simultaneous treatment with omalizumab and ciclosporin, she said.
Source: FomF (D) Dermatology and Allergy 2020
Literature:
- Zuberbier T, et al: The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018; 73(7): 1393-1414.
- Altrichter A: Angioedema and urticaria – What do I have to consider? Dr. med. univ. Sabine Altrichter. Dermatology and Allergology Refresher, Hofheim (D), 12.09.2020.
- Specialized information: https://compendium.ch
- Gericke J, et al: Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol 2017; 139(3): 1059-1061.e1.
- Metz M, et al: Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcεRI-positive cells in the skin. Theranostics 2017; 7(5): 1266-1276.
- Wedi B, et al: Urticaria. JDDG 2014; 12(11): 997-1010.
- Zuberbier T, et al: The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy 2014; 69: 868-887.
DERMATOLOGIE PRAXIS 2020; 30(6): 42-43 (published 8/12/20, ahead of print).