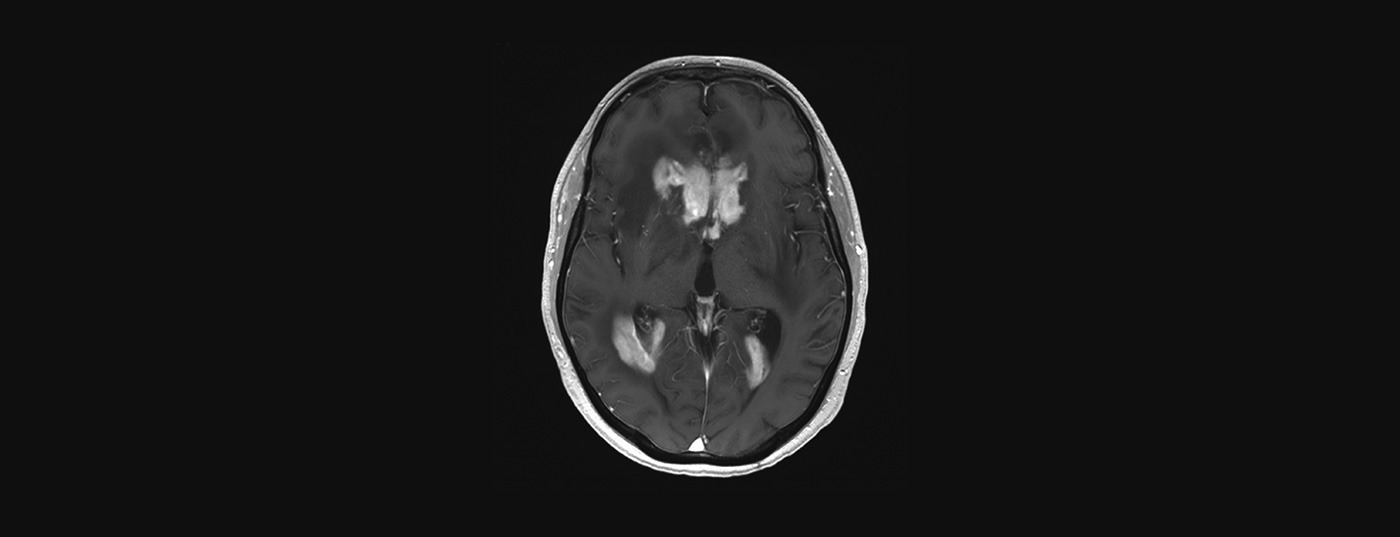
Therapy for primary CNS lymphoma has evolved greatly in recent years. The life expectancy of patients has increased. Methotrexate remains the basis of all induction therapies. In consolidation, the situation is somewhat less clear.
Primary CNS lymphomas (PZNSL) are extranodal variants of non-Hodgkin lymphomas that arise in a limited fashion in the brain, spinal cord, leptomeninges, or eyes and in which there is no systemic involvement. PZNSL are highly aggressive. They generally respond to chemotherapy and radiation therapy, with a good chance of remission. However, the risk of recurrence is high and the prognosis is poor in this case. PZNSL may occur in the context of immunosuppression (HIV/AIDS, congenital, post organ transplant) or in immunocompetent patients. This summary addresses the latter patients.
Epidemiology and prognosis
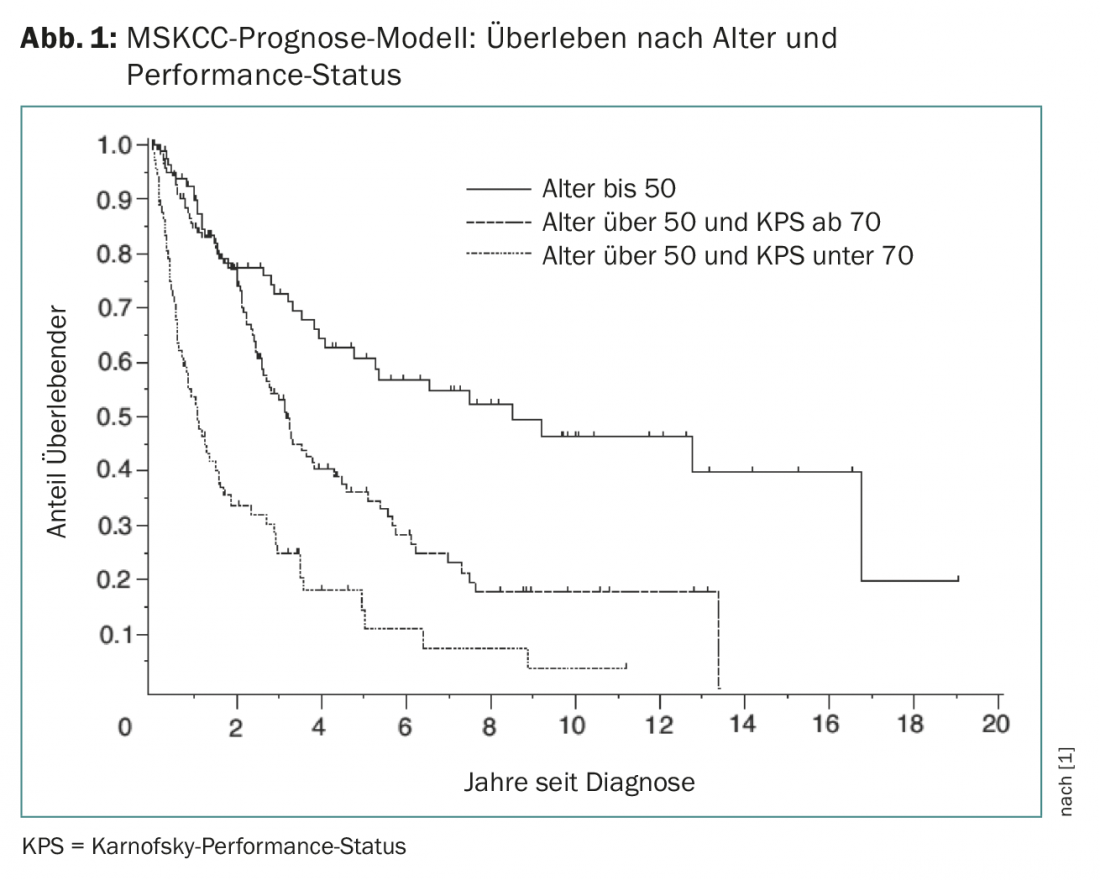
PZNSL is a rare disease with an incidence of 0.4-0.5:100,000 per year. It accounts for approximately 4% of all diagnosed brain tumors and 4-6% of all extranodal lymphomas and can occur in all age groups. The median age at diagnosis is 65 years. The Memorial Sloan Kettering Cancer Center (MSKCC) model can predict outcome based on Karnofsky performance status and age (Fig. 1) [1].
Clinic
The clinic can vary extremely depending on the patient and the location of the involvement in the brain. Focal neurologic deficits are seen in up to 70% of patients. Up to 43% of all patients show psychiatric abnormalities or behavioral disorders. Signs of increased intracranial pressure are also common. When the eyes are affected (vitreous infiltration), patients complain of blurred vision or unclear vision in 20-25% of cases.
Diagnosis and staging
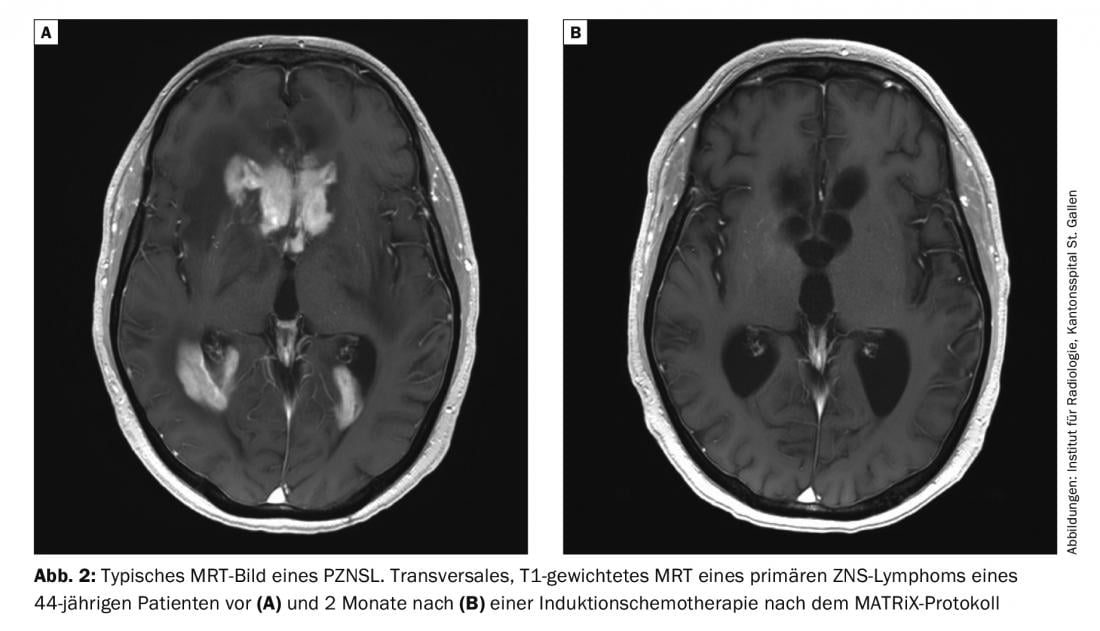
MRI with and without contrast is the investigation of choice for suspected PZNSL. Typically, centrally located periventricular solitary or multifocal lesions appear hyperintense on T2-weighted images with homogeneous contrast uptake and diffusion restriction (Fig. 2). Despite the typical clinical presentation and MRI imaging, the definitive diagnosis must be confirmed with brain biopsy. Steroids are lymphotoxic and their administration prior to biopsy may result in a false-negative diagnosis. Leptomeningeal involvement may occur in up to 20% of cases, and lumbar puncture should be performed unless contraindicated (intracranial pressure) to determine cell count, protein content, glucose, cytology, flow cytometry, and clonal “immunoglobulin rearrangement.” An eye examination with slit lamp is also part of the clarification (also in patients without visual disturbances). Once PZNSL is histologically confirmed, staging with whole-body PET should be performed. At a minimum, however, a computed tomogram of the lungs, neck, and abdomen and testicular ultrasonography should be requested if PET cannot be performed. Furthermore, a bone marrow biopsy should take place. A blood count including differential, serum LDH, and HIV screening are also part of the staging.
Newly diagnosed PZNSL
Modern treatment of a PZNSL is based on age, pre-existing conditions and general condition. Generally, induction therapy is started to achieve complete remission. This is followed by consolidation therapy, which is designed to eliminate residual microscopic disease and ensure long-lasting remission. Due to the lack of phase III trials comparing the different therapeutic regimens, various induction and maintenance therapies are used worldwide. What they all have in common, however, is the obligatory proportion of methotrexate (MTX). Other chemotherapeutic agents and the role and timing of radiation therapy are sometimes highly controversial. MTX in doses >1.5 g/m2 and in short infusion time also crosses the intact blood-brain barrier (high-dose MTX; HD-MTX). Since these doses are very toxic to the other organs, it is essential to co-apply leucovorin. Leukovorin inactivates MTX at the systemic level but cannot cross the blood-brain barrier, thus ensuring a high concentration of MTX in the CNS. MTX as monotherapy (8 g/m2) shows response rates (“overall response rate”, ORR) of 74% [2]. The benefit of polychemotherapy was demonstrated by Ferreri et al. in a randomized trial, where the addition of cytarabine to MTX resulted in a longer progression-free survival (PFS) of 18 vs. three months and an ORR of 69% vs. 40% compared with the standard arm [3].
It is common to include the anti-CD20 antibody rituximab in MTX polychemotherapy, although this large antibody molecule is likely to cross only the disrupted blood-brain barrier in contrast-receptive lesions, where it can exert its B-cell-depleting effect. A randomized trial of 227 patients (IELSG32) showed that the best ORR was observed when patients were treated with rituximab/thiotepa/HD-MTX/cytarabine (MATRiX regimen; ORR 86%). For comparison, the combinations of rituximab/HD-MTX/cytarabine or HD-MTX/cytarabine achieved an ORR of 73% and 53%, respectively [4].
The choice of induction therapy varies by region and physician. Typical therapies consist of rituximab/HD-MTX/procarbazine/vincristine (R-MVP), rituximab/HD-MTX, temozolomide/HD-MTX, MATRiX (see above), and rituximab/HD-MTX/teniposide/carmustine/methylprednisolone (R-MVBP).
These therapeutic regimens are generally well tolerated and even elderly patients can be treated with them as long as renal function is well preserved. Hepatitis, bone marrow failure and renal failure are possible side effects. Neurologic toxicity of MTX has been described, particularly as acute or subacute encephalopathies and multifocal leukoencephalopathies that develop months to years after MTX administration.
The role of whole-brain radiotherapy (WBRT) remains controversial: a phase III trial showed that consolidative WBRT prolonged PFS from 12 to 18 months, but without improving survival (32.4 vs. 37 months). However, increased neurotoxicity was also observed due to irradiation [5]. Nevertheless, several research groups continue to work with therapeutic regimens that include modified and, most importantly, dose-reduced WBRT. WBRT is increasingly being replaced by consolidation regimens with chemotherapy, as organ function and the patient’s general condition permit, because of its known and irreversible neurotoxicity. A multicenter study of 202 patients showed similar success rates for patients treated with high-dose etoposide/cytarabine after R-MTX induction therapy compared with WBRT [6]. More aggressive myeloablative chemotherapy with autologous stem cell transplantation (HCD-ASCT) can be performed in younger patients in good general health. Two studies using either R-MVP or HD-MTX/thiotepa/cytarabine as induction regimens showed high response rates (>90%) and PFS of >74 months with HCD-ASCT [7,8].
Several retrospective studies showed no benefit for the addition of intrathecal chemotherapy [9]. It is assumed that MTX doses >3 g/m2 already reach cytotoxic CSF concentrations that make intrathecal administration unnecessary and no longer justify the higher expense of therapy due to repeated lumbar punctures and the resulting risk of infection.
Monitoring after therapy
Since the risk of recurrence after first-line therapy is high, patients must be monitored regularly during the course. In addition to a clinical and neurological examination, cerebral imaging by MRI should be performed every three months for the first two years and every six months thereafter. After five years, the inspections can take place annually.
Elderly patients
The optimal management of elderly patients with PZNSL remains controversial. These patients present with specific problems, mostly due to their comorbidities, poorer general health, increased complication rates, and the fact that they typically respond less well to therapies. A French study compared two therapeutic procedures in patients over 70 years of age. This study showed that HD-MTX/procarbazine/vincristine/cytarabine was as well tolerated as less aggressive therapy with HD-MTX and temozolomide. However, ORR (82% vs. 71%) and overall survival (31 vs. 14 months) were better in the first group [10].
Therapy of relapsed or refractory PZNSL.
Although response rates after induction therapy are quite substantial, 10-15% of patients remain refractory to induction therapy. Furthermore, 50% of patients who responded to initial treatment develop a relapse during the course. The median time to recurrence is 10-18 months [11]. In the case of recurrence, the further prognosis is very poor. At the time of recurrence, repeat clinical staging with imaging of the entire CNS axis, ophthalmologic examination, and whole-body PET should be performed. It should be noted here that up to 10% of PZNSL patients show systemic recurrence.
To date, there is no standard therapy for this situation. The choice of therapy is based on the patient’s age, performance status, consideration of previous therapies and their response, and inclusion of comorbidities. Re-exposure to MTX may be a reasonable option, especially in patients who showed a long-term initial response to an MTX-containing induction regimen. This treatment yielded ORRs of 85-91% and median survival of 41-62 months [12,13]. Prospective studies with other chemotherapeutic agents, including temozolomide, pemetrexed, topotecan, or rituximab, showed response rates of 31-55% with median PFS rates of 1.6-5.7 months. In younger patients in good general health, HCD-ASCT may be an option [14]. WBRT remains an alternative in patients who cannot receive high-dose chemotherapy or are in very poor general health. Retrospective studies with WBRT show response rates of 74-79% and median survival of 10-16 months [15]. However, the leading symptom of cognitive deficits is often aggravated, so that very careful consideration must be given between the benefits and risks of WBRT. Also, in patients with severe cerebral preexisting conditions, a sensible treatment waiver should be considered.
New therapeutic strategies are also emerging. PD-1 ligand (“programmed cell death”; PDL-1) is deregulated and overexpressed in >50% of PZNSL. Therapies with monoclonal antibodies against PD-1 or PDL-1 such as nivolumab or pembrolizumab are thus promising. A small series of four patients with relapsed or refractory PZNSL showed a PFS of 14-17 months [16]. Prospective studies are currently in the recruitment phase. Lenalidomide, a thalidomide derivative, combined with rituximab showed a positive effect in a presentation at the 2016 ASCO meeting by Rubenstein et al. also showed a promising ORR of 67%.
Ibrutinib is an inhibitor of Bruton tyrosine kinase, a key part of the B-cell receptor signaling pathway that often has mutations in PZNSL. Ibrutinib crosses the blood-brain barrier and can be measured in the cerebrospinal fluid at therapeutic doses. In a small series of relapsed PZNSL, ibrutinib demonstrated an ORR of 77% with a PFS rate of 7.4 months [17]. A phase I trial combined initial ibrutinib therapy with consolidation consisting of temozolomide, etoposide, liposomal doxorubicin, dexamethasone, and rituximab. Patients in this study showed an ORR of 94% after ibrutinib, and 86% of patients subsequently achieved complete remission [18]. However, ibrutinib carries a high risk of pulmonary and cerebral aspergillosis.
Literature:
- Abrey LE, et al: Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006; 24(36): 5711-5715.
- Batchelor T, et al: Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol 2003; 21(6): 1044-1049.
- Ferreri AJ, et al: High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009; 374(9700): 1512-1520.
- Ferreri AJ, et al: Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016; 3(5): e217-227.
- Korfel A, et al: Randomized phase III study of whole-brain radiotherapy for primary CNS lymphoma. Neurology 2015; 84(12): 1242-1248.
- Rubenstein JL, et al: Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013; 31(25): 3061-3068.
- Omuro A, et al: R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015; 125(9): 1403-1410.
- Illerhaus G, et al: High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol 2016; 3(8): e388-397.
- Sierra Del Rio M, et al: Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol 2012; 106(1): 143-146.
- Omuro A, et al: Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol 2015; 2(6): e251-259.
- Jahnke K, et al: Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol 2006; 80(2): 159-165.
- Pentsova E, Deangelis LM, Omuro A: Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol 2014; 117(1): 161-165.
- Plotkin SR, et al: Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 2004; 10(17): 5643-5646.
- Soussain C, et al: Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: J Clin Oncol 2008; 26(15): 2512-2518.
- Hottinger AF, et al: Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology 2007; 69(11): 1178-1182.
- Nayak L, et al: PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017; 129(23): 3071-3073.
- Grommes C, et al: Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017; 7(9): 1018-1029.
- Lionakis MS, et al: Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017; 31(6): 833-843.e5.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(3): 34-38.