The main problem of angioplasty is recurrent stenosis, which in a large percentage is due to the development of neointimal hyperplasia (NIH). This can be effectively suppressed by applying antiproliferative drugs to stents and balloons. Initial studies on the topic show encouraging results, with further randomized trials expected to provide more information on potential side effects, patient selection, and also regarding the cost-benefit ratio.
Numerous epidemiological studies using objective examination techniques show a prevalence of peripheral arterial disease (PAVD) of 3-10% and 15-20% above the age of 70 years. Clinically, PAVK presents in stages from asymptomatic patients to critical limb ischemia (CLI). After the diagnosis of CLI, 30% of patients suffer amputation in the first year, and 20% have persistent pain and ulceration [1]. One-year mortality here is 20%, with cardiac and cerebral events dominating the fate of patients. Revascularization is therefore a primary therapeutic goal. The technical success of percutaneous transluminal angioplasty (PTA) is generally very high. For acute events such as flow-limiting dissections and the “elastic recoil” of the artery, the stent provides a mechanical solution in terms of vascular support. The main problem of the method is the occurrence of recurrent stenosis, which occurs in 30-50%/year depending on the lesion length and localization. The main cause of recurrent stenosis is the development of neointimal hyperplasia (NIH). This can be effectively suppressed by topical application of antiproliferative drugs. In percutaneous coronary angioplasty, this concept has been successfully applied for almost a decade and is widely supported by several randomized trials. In contrast, the evidence on this method is much lower in the peripheral current area. A review article on the topic was already published in VASA 02/2012 [2]. The current study results were added below.
Recurrent stenosis after angioplasty
NIH is the result of a vasoproliferative cascade that begins in vascular trauma by PTA with endothelial damage and platelet activation. The complex biochemical mechanisms involved in NIH development are not yet fully understood. Secondary events such as oxidative stress and inflammation cause the expression of various matrix metalloproteinases (MMPs) via an increase in free radicals, which contribute to the degradation of collagen and elastin in the arterial wall, allowing the migration of cells into the vascular intima. The paradigm that these cells are solely vascular medullary smooth muscle cells that have lost desmin expression and contractility and now express vimentin has been challenged by research from several groups. It has been demonstrated that after coronary angioplasty or bypass surgery, fibroblasts also migrate from the adventitia through the media into the intima, thereby acquiring the expression of SMA (“smooth muscle actin”) and transforming it into myofibroblasts [3–5]. After PTA of dialysis shunts, smooth muscle cells and endothelial cells derived from circulating stem cells from bone marrow were also detected [6]. From a therapeutic point of view, this provides a promising basis for new therapeutic approaches.
Once in the vascular intima, myofibroblasts proliferate and synthesize extracellular matrix proteins. This process typically ends approximately three to four months after vascular trauma in a neointimal cell layer, which, depending on its thickness, can constrict the vascular lumen such that recurrent stenosis occurs. Application of antiproliferative cell cycle inhibitory drugs to balloons and stents (paclitaxel, sirolimus, everolimus) effectively suppresses the formation of the neointimal layer. Topical administration allows high local drug concentrations without potentially causing systemic side effects.
Sirolimus (rapamycin) is a natural macrolide with immunosuppressive activity. It is able to increase the concentration of the cell cycle inhibitory protein p27, inhibiting the transition from G1 to S phase of the cell. Everolimus, which is a derivative of sirolimus, has the same mechanism of action. The active ingredient Paclitaxel (PTX) was originally isolated from the bark of the Pacific yew tree (Taxus brevifolia). Recently, it can be partially synthesized from the European yew tree. Paclitaxel irreversibly binds to beta-tubulin, preventing microtubule degradation, which is why the cell can no longer divide mitotically. Paclitaxel is highly lipophilic and is therefore rapidly absorbed into the vascular wall.
Study situation
The studies on drug-eluting stents (DES) and drug-coated balloons (DCB) generally distinguish between applications to the caliber femoropopliteal arteries ( Table 1) and to the correspondingly narrower below-the-knee arteries (BTK) (Table 2).
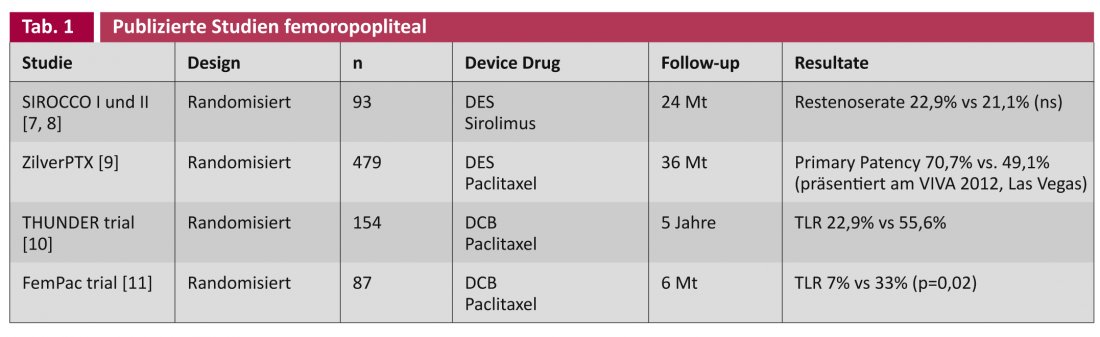
With the SIROCCO studies, already in 2002 resp. 2006, a sirolimus-eluting stent was randomly compared against the same uncoated stent in the treatment of the superficial femoral artery [7, 8]. After two years, no difference was found in the occurrence of recurrent stenosis, possibly because of the sirolimus concentration chosen to be too low (90 μg/cm2). The recent prospective randomized multicenter ZilverPTX® stent trial compared a paclitaxel-eluting nitinol stent (3 μg/mm2) versus plain old balloon angioplasty (POBA) alone and the same uncoated bare metal stent (BMS), respectively, in 479 patients. The study was designed for five years, with interim results being continuously reported at congresses: After currently three years, the coated stent shows a statistically significant marked superiority in primary patency (= “angiographic or duplex sonographic absence of recurrent stenosis”) of 70.7% vs. 49.1% (presented VIVA 2012, Las Vegas).
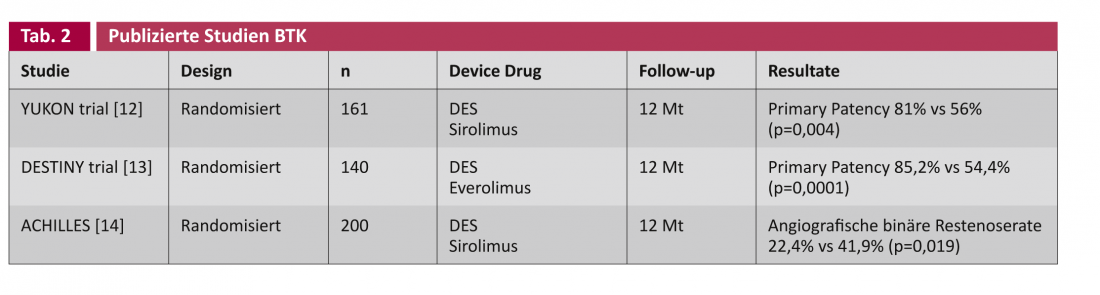
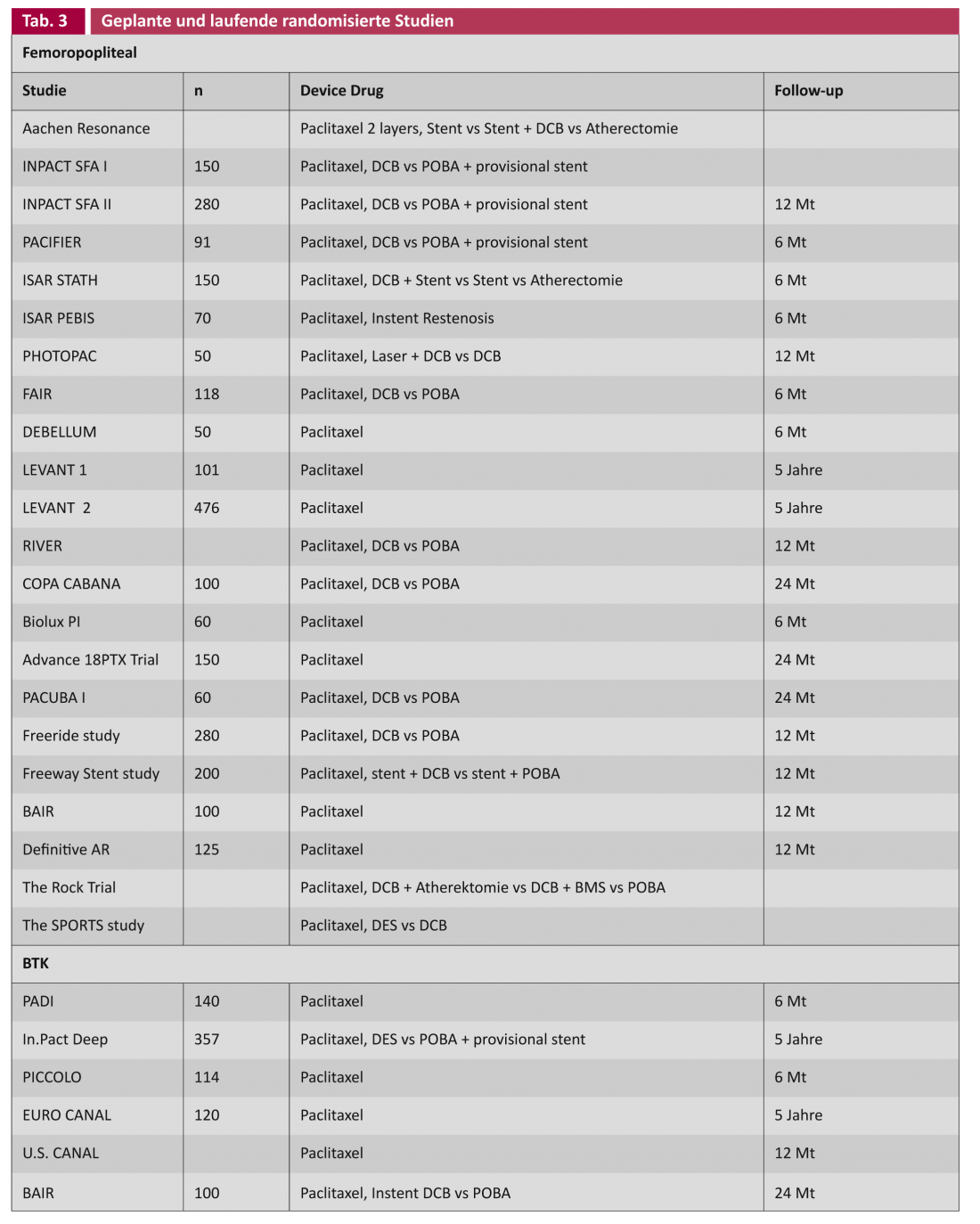
In principle, it is desirable in the treatment of vascular lesions if no foreign material such as a stent has to be left behind. The DCB concept takes this into account. In the THUNDER trial, the target lesion revascularization (TLR) rate showed a significant advantage for DCB even after five years, albeit with only short vascular lesions (Leipzig Interventional Course 2012). The key question remains whether there is a place for the DCB in long and heavily calcified lesions. Studies investigating DCB in conjunction with other technologies such as atherectomy, as well as a whole series of DCB studies in the primary setting, are ongoing (Table 3).
The application of “drug-eluting” technology in the infrapopliteal arterial segment requires separate consideration. Patients with critical limb ischemia usually have multiple lower leg artery obstructions, as seen in diabetics and patients with severe renal insufficiency. By ensuring at least one open vessel to the foot (“straight line flow”), wound healing should be improved and the amputation rate reduced. However, angioplasty of the transtibial arteries alone often produces an insufficiently good dilatation result, not least because of the length of the vessel occlusions. Dissection and recoil often occur here, potentially leading to early occlusion in the fine-caliber arteries.
Scheinert et al. were the first to deploy a drug-eluting coronary stent infrapopliteally in 30 patients in 2006. After six months, not a single recurrent stenosis was found [15]. The YUKON-BTK trial randomized this to a sirolimus-eluting stent and also found a statistically significant superiority of the DES in primary patency (81% vs. 56%, p=0.004) [12]. Other published randomized trials on the topic include DESTINY [8] and ACHILLES [14], which also find this statistically significantly better primary patency for the DES.
However, with respect to the important clinical endpoints of amputation and death, the technology does not yet provide a demonstrable benefit, as suggested by Rastan et al. in a review of pooled data from 1039 patients in the above studies [16].
Discussion
In addition to the randomized trials listed above, a whole range of registry data exists [2]. Interpretation of the results suggests that drug coating, analogous to coronary angioplasty, is a promising method in the treatment of PAVK. Increasing evidence shows that the rate of recurrent stenosis, and therefore potentially reinterventions, can be reduced both femoropopliteally and infrapopliteally. However, it is premature to formulate a gold standard from this, especially since randomized trials with inclusion criteria do not always reflect regular everyday conditions. The results of ongoing large studies will provide more information on potential side effects, on proper patient selection, and also regarding the cost-benefit ratio (Tab. 3). The latter requires special attention, because the potential reduction of the reintervention rate is expected to reduce costs, as has been shown comparatively in cardiology studies by the use of DES [17].
No prospective randomized data exist in posttreatment using dual antiaggregation. According to the Swiss consensus, dual antiaggregation is generally performed for one month after peripheral BMS implantation [18]. Because of delayed reendothelialization after DES/DCB implantation, this is performed for a longer time, analogous to cardiology. In cited studies, this was between one and six months depending on the vascular region treated.
Literature:
- Norgren L, et al; TASC II working group: J Vasc Surg 2007; 45 Suppl: S5-67.
- Buechel R, Stirnimann A, Zimmer R, Keo H, Groechenig E: VASA 2012; 41(4): 248-261.
- Scott NA, et al: Circulation 1996; 93(12): 2178-2187.
- Shi Y, et al: Circulation 1996; 94(7): 1655-1664.
- Shi Y, et al: Circulation 1997; 95(12): 2684-2693.
- Sata M, et al: Nat Med 2002; 8(4): 403-409.
- Duda SH, et al: Circulation 2002; 106(12): 1505-1509.
- Duda SH, et al: J Endovasc Ther 2006; 13(6): 701-710.
- Dake MD, et al; on behalf of the Zilver PTX Investigators: Circ Cardiovasc Intervent 2011; 4(5): 495-504.
- Tepe G, et al: NEJM 2008; 358(7): 689-699.
- Werk M, et al: Circulation 2008; 118(13): 1358-1365.
- Rastan A, et al: Eur Heart J 2011; 32: 2274-2281.
- Bosiers M, et al: J Vasc Surg 2012; 55(2): 390-398.
- Scheinert D, et al; ACHILLES Investigators: J Am Coll Cardiol 2012; 60(22): 2290-2295.
- Scheinert D, et al: EuroIntervention 2006; 2(2): 169-174.
- Rastan A, Noory E, Zeller T: Vasa 2012; 2: 90-95.
- Filion KB, et al: Am J Cardiol 2009; 103(3): 338-344.
- Jäger K, et al: Switzerland Med Forum 2009; 9(39): 690.
CARDIOVASC 2013; 12(1), 15-19