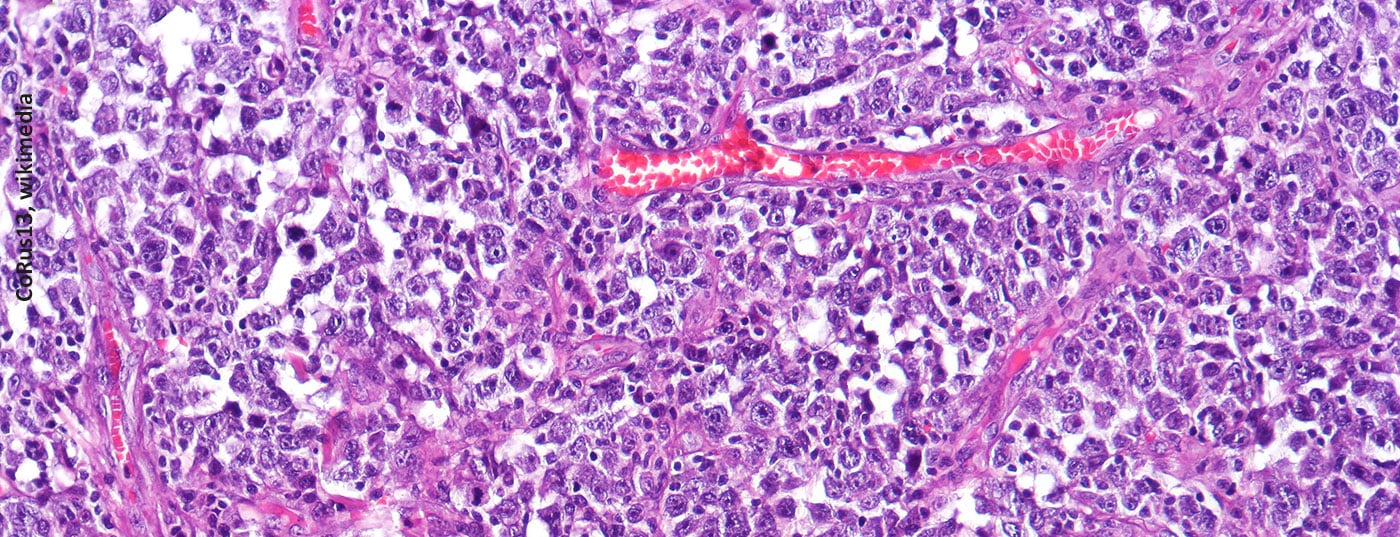
Diffuse large B-cell lymphoma is the most common type of non-Hodgkin lymphoma. If left untreated, accumulations of lymphoma cells impede the function of affected organs and lead to the death of patients within a few months. The treatment strategy depends on the stage of the disease and the course to date, as well as on general patient characteristics. For adults with relapsed or refractory DLBCL who are not eligible for hematopoietic stem cell transplantation, an antibody-drug conjugate became available last year as an add-on treatment option.
In Switzerland, approximately 1500 people develop non-Hodgkin’s lymphoma (NHL) each year [1]. The incidence increases in an age-correlated manner and is approximately 2-3 per 100,000 population in the 30-year-old age group, whereas it is 70-120 per 100,000 population in the 80-year-old age group. Histologically, NHL are divided into the more common B-cell lymphomas and the less common T-cell lymphomas. The etiology of NHL is unknown, but there is evidence of an association with viral diseases (e.g., Epstein-Barr virus, HIV, hepatitis B, hepatitis C). Bacteria such as Helicobacter pylori also increase the risk of lymphoma. Because diffuse large B-cell lymphoma (DLBCL) progresses rapidly and spreads lymphoma cells throughout the body even in early stages of disease, it is considered an aggressive lymphoma [2,3]. The REAL classification is used to define lymphomentities according to morphologic, clinical, immunophenotypic, and molecular genetic markers [3,4].
Diagnostic classification according to the Lugano criteria
Clinically, the disease usually manifests as rapidly progressive lymphadenopathy, extranodal manifestations, and general symptoms [6]. In general, the enlarged lymph nodes are not painful, in contrast to the tender nodes that often occur with viral infections. If there are positive palpatory findings of enlarged superficial lymph nodes in conjunction with pathologic laboratory tests (e.g., abnormal leukocyte count and pathologic lactate dehydrogenase [LDH]-value), the suspicion can be substantiated by ultrasonography of superficial lymph nodes or computed tomography (CT) [3]. These two imaging techniques are also well suited for targeted lymph node biopsy if lymph node resection is not to be performed. Subsequently, further diagnostics are performed predominantly by means of cross-sectional imaging techniques, in particular the [18F]FDG-PET/CT. PET/CT has largely replaced contrast-enhanced CT as the recommended measure in this regard and is firmly established in clinical guidelines, such as the ICML (International Conference on Malignant Lymphoma) Lugano classification [3,7,8].

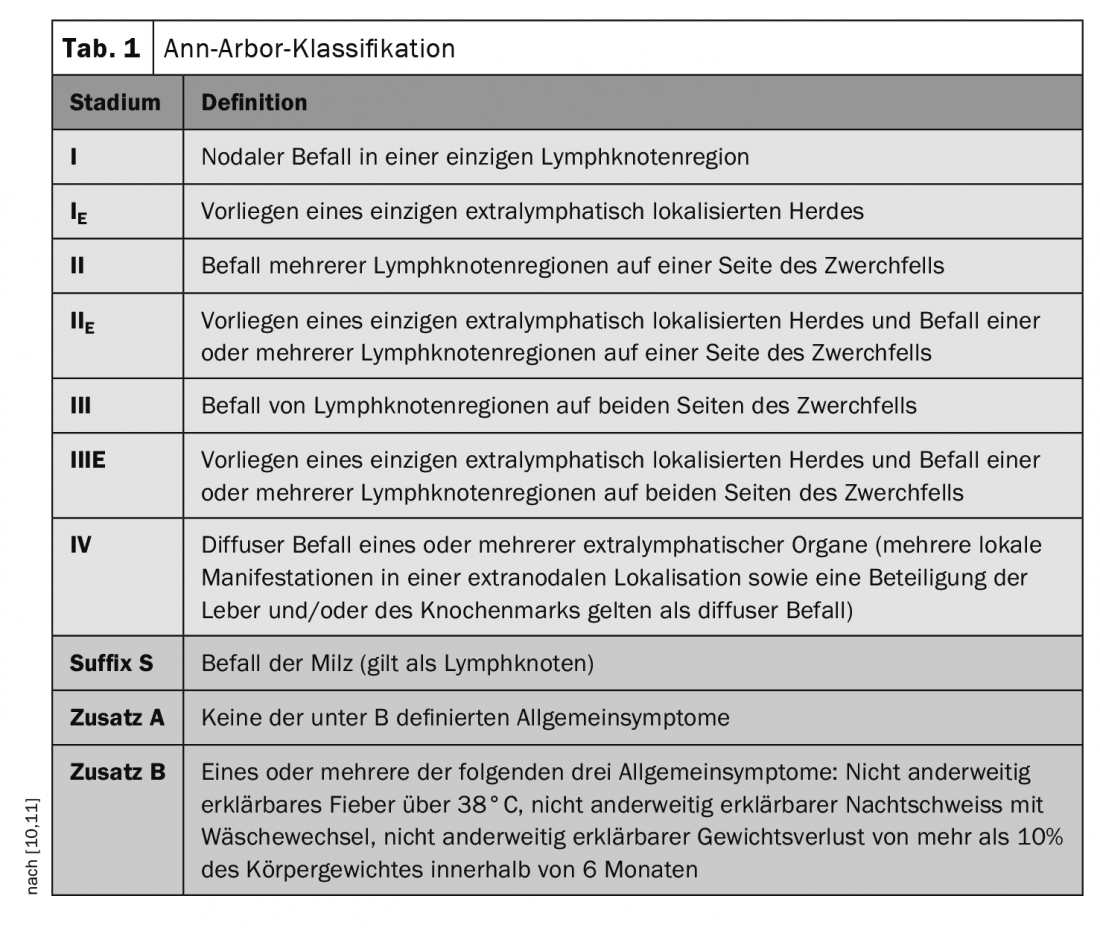
Imaging not only has an important role in the diagnosis of pathologic lymph nodes and organ manifestations of Hodgkin’s and non-Hodgkin’s lymphomas, but also plays an important role in disease progression and follow-up (Box) [9]. Assignment to a disease stage (stages I to IV) is made in DLBCL according to the Ann-Arbor classification (Table 1) [10,11]. From one lymph node, the malignant cells can migrate via the lymphatic vessels to neighboring lymph nodes and continue to multiply there. Only in later stages of the disease course do the DLBCL cells invade the blood system and can then also affect non-lymphatic organs.
The German Lymphoma Alliance’s Aggressive Lymphoma Working Group, led by Prof. Björn Chapuy, MD, Charité Universitätsmedizin Berlin, Germany, has published a revised brochure on diffuse large B-cell lymphoma.
It is available for download free of charge for physicians and patients at https://lymphome.de [5].
Add-on treatment with antibody-drug conjugate
If left untreated, DLBCL is rapidly fatal, so the diagnosis is an indication for initiating therapy. The intention of therapy is primarily curative, even in patients of advanced age and/or with comorbidity. The standard of care is chemoimmunotherapy R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). However, approximately 40% of patients have refractory or recurrent disease [6]. For these, the antibody-drug conjugate polatuzumab vedotin (Polivy®) has been available since last year. This is an antibody against CD79b, a subunit of the B-cell receptor, bound to the mitosis inhibitor monomethyl auristatin-E. In combination with bendamustine and rituximab, polatuzumab vedotin is indicated for the treatment of adults with relapsed or refractory DLBCL who are not eligible for hematopoietic stem cell transplantation [12].

Higher PFS and OS in study arm with polatuzumab vedotin
The approval of Polivy® is based on the randomized, open-label Phase Ib/II study GO29365 [13–15]. In this study, the efficacy, safety, and tolerability of polatuzumab vedotin as an add-on to bendamustine plus rituximab (BR) were compared with BR alone. The study included a randomized cohort of patients with pretreated DLBCL who were not eligible for autologous hematopoietic stem cell transplantation and had relapsed or refractory disease after at least one prior systemic chemotherapy regimen. Among the 80 patients randomly assigned to treatment with Polivy plus BR (n=40) or BR alone (n=40), the median age was 69 years (range: 30-86 years). 98% of patients had not otherwise specified (NOS) DLBCL. Overall, 48% of patients had ABC (activated B-cell) DLBCL and 40% had GCB (germinal center B-cell like) DLBCL.

The 1:1 randomized study participants received either Polivy® plus BR or BR alone for six 21-day cycles [9] (box). The primary endpoint of the study was the complete response (CR) rate at the end of treatment (6-8 weeks after day 1 of the 6th treatment cycle or the last study treatment). This was assessed by PET-CT by an independent review committee (IRC). The results show that 40% of patients treated with Polivy® plus BR achieved a complete response (n=16/40), meaning that no cancer cells were detected at the time of study, compared to 17.5% (n=7/40) treated with BR alone. Moreover, a median progression-free survival (PFS) of 7.6 months was achieved in the study arm with polatuzumab vedotin as an add-on compared with 2.0 months in the control arm (95% CI: 6.0-17.0 and 95% CI: 1.5-3.7, respectively). Median overall survival (OS) was 12.4 months in participants treated with polatuzumab vedotin plus BR versus 4.7 months in the control arm.
Literature:
- Wintertur Cantonal Hospital, www.ksw.ch/gesundheitsthemen/lymphsystem/non-hodgkin-lymphom-nhl/, (last accessed 03.03.2022).
- Malignant Lymphoma Competence Network,https://lymphome.de/diffus-grosszelliges-b-zell-lymphom (last accessed 03.03.2022)
- Mayerhöfer ME, Haug A: Hybrid imaging in lymphoma. Radiologist 2020; 60: 376-385.
- Harris NL, et al: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994; 84: 1361-1392.
- “New: Brochure on “Diffuse large B-cell lymphoma (DLBCL)” with current treatment strategies,” Science Information Service, Jan. 05, 2022; https://nachrichten.idw-online.de (last accessed Mar. 03, 2022).
- Siegmund-Schultze N: Advanced diffuse large B-cell lymphoma: polatuzumab vedotin prolongs progression-free survival in the first-line setting. Dtsch Arztebl 2022; 119(5): A-190/B-157
- Cheson BD, et al: Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32: 3059-3068.
- Barrington SF, et al: Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014; 32: 3048-3058.
- Tumor Center Upper Austria, Medical Guideline, www.tumorzentrum.at/fileadmin/user_upload/20210928_Leitlinie_DLBCL.pdf (last accessed 03.03.2022).
- Onkopedia, www.onkopedia.com/de/onkopedia/guidelines/diffuses-grosszelliges-b-zell-lymphom/@@guideline/html/index.html.
- Lister TA, et al: Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds Meeting. J Clin Oncol 1989; 7: 1630-1636.
- Swissmedicinfo: Medicinal product information, www.swissmedicinfo.ch (last accessed 03.03.2022).
- Sehn L, et al: Polatuzumab Vedotin Plus Bendamustine and Rituximab in Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Updated Results of a Phase Ib/II Randomized Study and Preliminary Results of a Single-Arm Extension. ASH December 2020. abstract #3020 and poster
- European Medicines Agency (EMA), Product information: Polivy® www.ema.europa.eu/en (last accessed 03.03.2022).
- “Swissmedic granted marketing authorization for Roche’s Polivy for patients with pretreated aggressive lymphoma,” Roche, 7/20/2021.
- ADC Review. What are antibody-drug conjugates? https://adcreview.com/adc-university/adcs-101/antibody-drug-conjugates-adcs (last call 03.03.2022)
HAUSARZT PRAXIS 2022, 17(3): 44-46
InFo ONCOLOGY & HEMATOLOGY 2022, 10(2): 38-39.