At the DGIM Congress, the various therapeutic options for irritable bowel syndrome were discussed. Apparently, German general practitioners mainly favor probiotics, spasmolytics and phytotherapeutics in treatment. What other measures are available?
Current understanding is that in IBS, a disturbance of the gut-brain axis leads to increased visceral pain perception and gut motility. An alteration of the microbiome appears to play a causative role. Irritable bowel syndrome is typically accompanied by bloating, diarrhea or constipation, and abdominal pain. Depressive moods often accompany the symptoms.
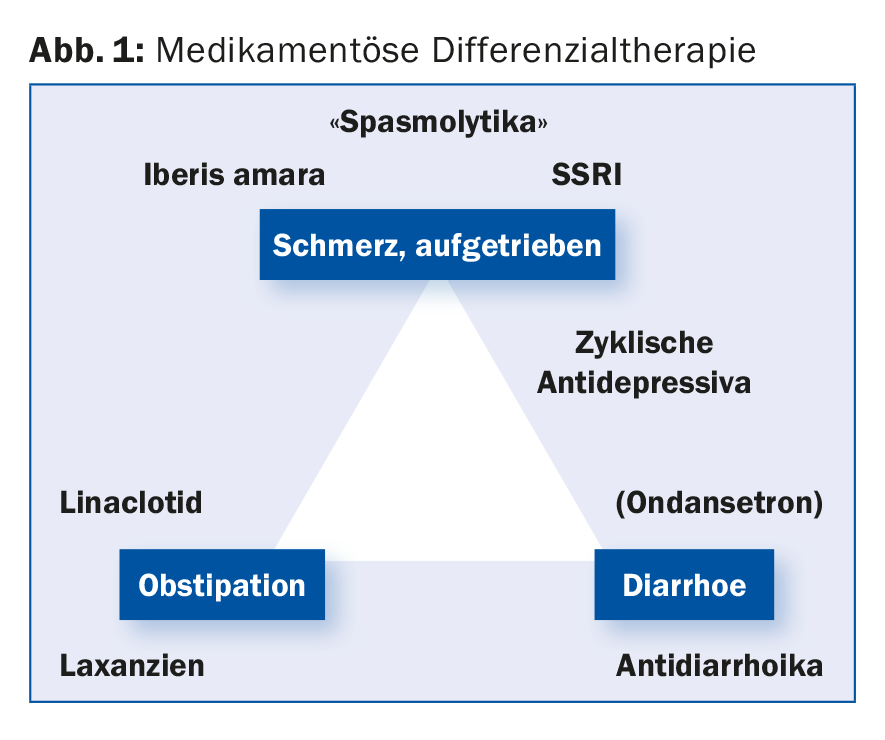
According to a survey presented as a poster at the congress, German family physicians prefer treatment with probiotics, spasmolytics and phytotherapeutics. The primary care providers thus considered the three drug classes to be the most effective, for which there is the weakest evidence in studies, according to Prof. Stefan Müller-Lissner, MD, Park-Klinik Weissensee, Berlin. The vagueness in the diagnosis of IBS (32 different diseases) and in its drug differential therapy (Fig. 1) does not facilitate the management of affected patients. Irritable bowel syndrome does not have a uniform pathophysiology, which is already suggested by the classification into the subtypes irritable bowel syndrome of the diarrhea-dominant type and constipation-dominant type or with alternating stool behavior.
However, IBS is associated with defined peripheral and systemic disorders, said Prof. Dr. Michael Schemann, Technical University of Munich, at the annual meeting of the German Society of Internal Medicine (DGIM). We know about the disturbed signaling pathways in the brain, but this does not mean that the disease only occurs in the CNS. Examples of organic (measurable) correlates in IBS are motility disorders of the bowel or bile acidosis syndrome. With the help of biomarkers, it is now possible to identify RDS subtypes and treat them more specifically. Biomarkers of treatable pathology currently measurable are 48-h colonic transit (motility disorder) and proteases in the mucosal supernatant (peripheral sensorimotor disorders). Analysis of stool samples or measurement of immune mediators may be considered as additional biomarker candidates. However, these markers are not routinely used.
Therapy: medication, diet and probiotics
The condition for the occurrence of irritable bowel syndrome is the patient’s affective evaluation of the symptoms. “It needs a peripheral signal as an initial trigger,” Prof. Müller-Lissner said. Triggers may include (abnormal) motility, certain food components, bacteria, or gas formation. These triggers can also be used to classify the different therapeutic approaches: Diet, probiotics and drug therapies. Overall, there is no breakthrough therapy; in pain modulation, tricyclic antidepressants or SSRIs provide relief, with the disadvantage that they must be taken long-term (rather than “on-demand” like other therapeutics), the expert said.
Diet: dietary fiber and FODMAP
The recommendation to favor a high-fiber diet, for example with wheat bran and psyllium husks, has so far shown only a borderline benefit in studies, says Prof. Müller-Lissner. In recent years, the so-called FODMAP diet has emerged as more effective, i.e. a diet that avoids certain types of fruit and vegetables. The acronym stands for “Fructans, Oligosaccharides, Disaccharides, Monosaccharides (and) Polyols.” Such carbohydrates and sugar alcohols are present in many foods and, in healthy individuals, are almost completely broken down and absorbed in the small intestine. In irritable bowel patients, these may not be completely broken down and enter the colon. They increase the influx of water into the intestine and, once they reach the colon, are fermented by intestinal bacteria. This results in the formation of gas. The result can be unpleasant flatulence, diarrhea or unclear abdominal pain. FODMAP sources include broccoli, cauliflower, peas, potatoes, leeks, and onions, as well as apples, apricots, cherries, pears, prunes, and dried fruits.
Abnormal motility: spasmolytics, peppermint oil and laxatives.
Abnormal motility in the gastrointestinal tract may manifest as abdominal pain. As an old but less known spasmolytic, peppermint oil can be used in irritable bowel patients with good efficacy. This is due to the high bioavailability of peppermint oil. The anticholinergic hyoscine (Buscopan®) is about equally effective for treating cramping abdominal pain in irritable bowel syndrome. For mebeverine, on the other hand, meta-analyses have not demonstrated a significant effect.
Laxatives may be considered for constipation and bloating. For the guanylate cyclase C receptor agonist linaclotide, which is used to treat irritable bowel syndrome with constipation, an analgesic effect was additionally shown in hypersensitive mice.
Microbiommodulation: probiotics and antibiotics
Gas formation occurs when fiber is cleaved by microbiota; conversely, abnormalities in intestinal bacterial colonization have been found in patients with meteorism. Yet probiotics have been shown to have little effect on bloating and abdominal pain. The nonabsorbable antibiotic rifaximin emerged as superior to placebo in terms of amelioration of meteorism.
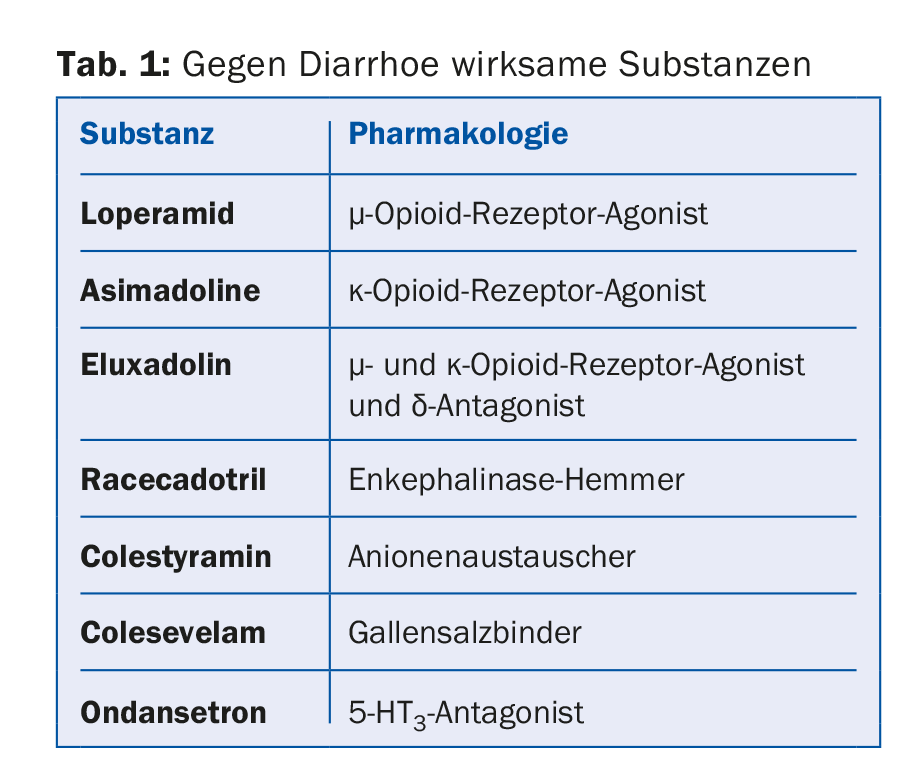
Diarrhea: loperamide and new agents (Table 1).
In addition to well-known antidiarrheal agents, bile acid binders are also useful in the treatment of irritable bowel syndrome. Indeed, bile acids stimulate motility and secretion in the colon, resulting in a shorter transit time and favoring diarrhea. Therapy with a bile acid binder such as colestyramine or colesevelam may delay colonic emptying in patients with diarrhea-type irritable bowel syndrome.
General irritable bowel symptoms: Iberis amara
For the herbal cocktail Iberogast®, consisting of the bitter ribbon flower (Iberis amara) and the other eight combination partners chamomile, caraway, lemon balm, peppermint, celandine, licorice, angelica and milk thistle, there is an original study showing an improvement in the parameter pain as well as global symptoms. The mechanism of action includes influencing the peripheral threshold of mucosal stimuli. The preparation is approved in the indication “irritable bowel syndrome”.
Source: Congress of the DGIM, April 14-17, 2018, Mannheim, Germany.
Further reading:
- Enck P, et al: Irritable bowel syndrome – dissection of a disease. A 13-steps polemic. Z Gastroenterol 2017 Jul; 55(7): 679-684.
- Ford AC, et al: American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014 Aug; 109(Suppl 1): S2-26.
- Wong BS, et al: Increased Bile Acid Biosynthesis Is Associated With Irritable Bowel Syndrome With Diarrhea. Clin
- Gastroenterol Hepatol 2012; 10: 1009-1015.
- Madisch A, et al: Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-center trial. Aliment Pharmacol Ther 2004 Feb 1; 19(3): 271-279.
HAUSARZT PRAXIS 2018; 13(5): 43-44











