Cardiovascular disease (CVD) remains the leading cause of death worldwide. Recent guidelines on HKE prevention highlight the extent to which elevated low-density lipoprotein cholesterol (LDL-C) is one of the most important contributors to atherosclerotic HKE. Accordingly, effective LDL-C lowering is indicated.
Cardiovascular disease (CVD) remains the leading cause of death worldwide [1]. The latest statistics from the World Health Organization (WHO) show that 17.9 million people die from HKE each year worldwide, accounting for an estimated 32% of all deaths. Of these, approximately 85% are due to myocardial infarctions (heart attacks) and strokes.
Recent guidelines on HKE prevention highlight the extent to which elevated low-density lipoprotein cholesterol (LDL-C) is one of the most important contributors to atherosclerotic HKE [2]. Because elevated LDL-C concentrations respond to pharmacological lowering, the relevant European and US guidelines for dyslipidemia have put forward treatment targets in different patient categories to emphasize the need for beneficial LDL-C lowering in patients at high risk of HKE or patients with existing HKE. Statins have been established as the gold standard for the treatment of cholesterol in primary and secondary prevention for nearly two decades. In recent years, ezetimibe has been the second drug added to statin treatment. Ezetimibe inhibits cholesterol uptake from the intestine, providing additional LDL-C lowering.
Despite the success of statins, registry data show that currently available therapies are underutilized. The NOR-COR (NORwegian CORonary) study published in 2017 showed that in patients who survived an acute myocardial infarction (heart attack), 57% had LDL-C levels above 1.8 mmol/l; 22% above 2.5 mmol/l; and 10% above 3.0 mmol/l, at a time point between 2 and 36 months after the heart attack [3]. In the POLASPIRE study, conducted in 2017-2018 as part of the EUROASPIRE V trial, target LDL-C levels were achieved by only 20% of women and 25% of men [4]. According to current guidelines, these patients should aim for an LDL-C level of 1.4 mmol/l or lower [2]. Achieving the current recommendations for even lower LDL-C concentrations is a major challenge in clinical practice.
Particular high-risk groups of pa-tients include those with familial hypercholes-te-rin-emia (FH). Because of high cholesterol levels from birth, patients with FH have a much higher risk of HKE than those with later-onset hypercholesterolemia [5]. Most patients with FH do not achieve their LDL-C treatment goals. In a Norwegian group of individuals, 25% of patients in primary prevention and only 8% of patients in secondary prevention achieved LDL-C targets of below 2.5 mmol/l and below 1.8 mmol/l, respectively, in treatment mainly with statins and ezetimibe [6]. As with secondary prevention, the 2019 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) joint guidelines for the management of dyslipidemia significantly lowered FH treatment targets to LDL-C <1.8 mmol/l in primary prevention and <1.4 mmol/l in secondary prevention, underscoring the need for new treatment options [7].
Another problem in preventive cardio-logy is the residual risk of recurrent events in patients with established HKE, despite current preventive therapies. Recently, the role that triglyceride-rich lipoproteins play in the development of atherosclerosis and HKE has been recognized [7]. These particles can mediate the accumulation of cholesterol within the arterial intima and trigger pro-inflammatory processes. While fibrates have significant triglyceride-lowering properties, their success in lowering cardiovascular events is limited, leading to interest in drugs that modify or reduce cardiovascular risks associated with these lipoproteins. Another factor that increases residual risk is elevated lipoprotein(a) [Lp(a)] concentration. Lp(a) is a genetic LDL-like particle characterized by adding apolipoprotein(a) [apo(a)] to an apolipoprotein B100-based particle. Up to 20% of the population have levels that could increase their risk of HKE. Large lipid-lowering therapies have little effect on Lp(a) concentrations, but new gene-based therapies that specifically target apo(a) are being developed.
In the following article, we will review the evidence for the major lipid-lowering agents that have been recently introduced or are still in development, following the introduction of statins and ezetimibe.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors
PCSK9 is an enzyme expressed in many tissues and cells. It binds to the LDL receptor on hepatocyte membranes, which targets the receptor for intracellular degradation in lysosomes. LDL receptors that are not bound to PCSK9 can be recirculated more than 100 times. Blocking PCSK9 leads to an increase in functional LDL receptors, increased transport of LDL particles from the extracellular to the intracellular space, and thus a decrease in blood LDL-C concentration (Fig. 1). The first two PCSK9 inhibitors, alirocumab and evolocumab, were approved by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) in 2015 as injections once every two weeks or once monthly. A recent meta-analysis in patients with atherosclerotic HKE but not FH, involving 66 478 patients in 39 randomized controlled trials, found that PCSK9 inhibitors achieved LDL-C reductions of approximately 60%. This reduction was associated with a lower risk of myocardial infarction (relative risk [RR], 0.80; 95% confidence interval [KI], 0.74-0.86; p<0.0001), ischemic stroke (RR, 0.78; 95% CI, 0.67-0.89; p=0.0005), and coronary revascularization (RR, 0.83; 95% CI, 0.78-0.89; p<0.0001) associated, compared with control values. However, the effects of PCSK9 inhibition on all-cause mortality and cardio-vascular death were not statistically significant during the median observation period of 2.3 years; a time frame that may have been too short to demonstrate effects on mortality. Meanwhile, the very latest PCSK9 work, a follow-up to one of the largest phase III trials, had an observation period of 8.4 years, and showed a significant 23% reduction in cardiovascular mortality compared with placebo [8]. The use of these PCSK9 inhibitors has not been associated with an increased risk of neurocognitive adverse events, elevated liver enzyme levels, rhabdomyolysis, or new-onset diabetes mellitus [8].
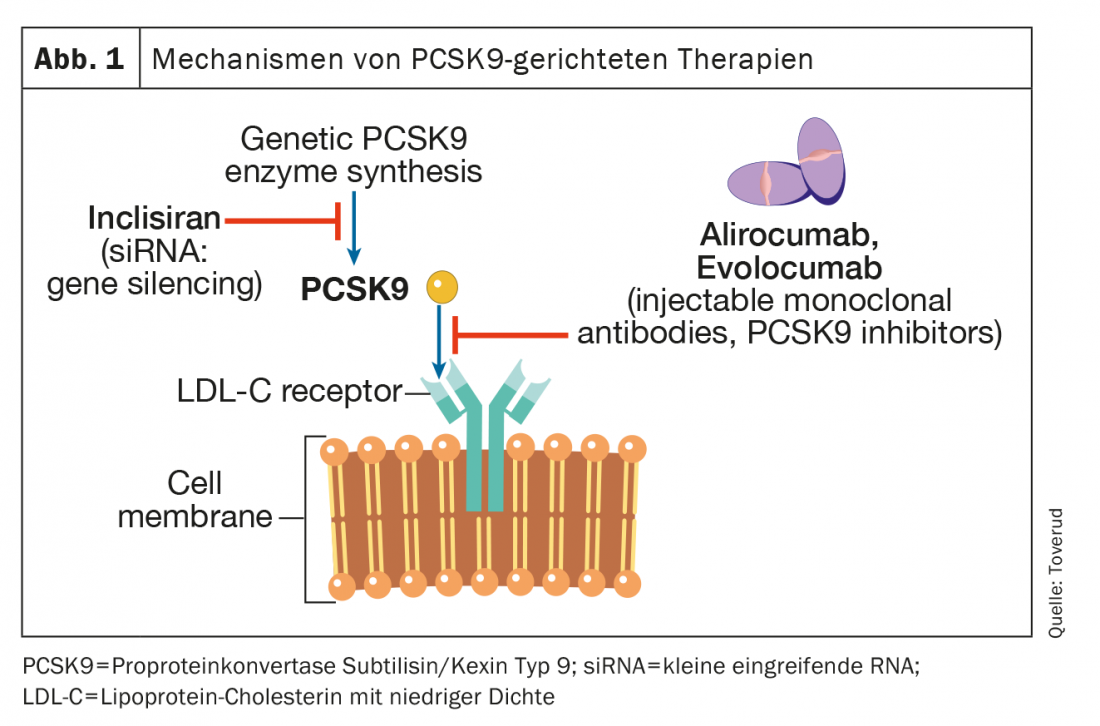
In randomized, double-blind trials of alirocumab and evolocumab in patients with heterozygous FH on stable lipid-lowering therapy, mainly with statins and ezetimibe, LDL-C concentrations were reduced by 50-60 to about 1.8 mmol/l [9,10]. Approximately 60% of patients can achieve LDL-C treatment goals of less than 1.8 mmol/l [8,9]. The results of long-term, open-label treatment trials with alirozumab and evolocumab have been encouraging, with sustained lowered LDL-C levels, low rates of discontinuation due to side effects, and low prevalence of anti-drug antibodies [11,12]. In the rare condition of homozygous FH (i.e. FH-causing mutations inherited from both parents), little or no residual LDL receptor activity remains. Therefore, drugs that act by upregulating LDL receptors, including statins and ezetimibe, have limited effect. PCSK9 inhibitors also upregulate LDL receptors, but in homozygous FH patients who have some residual LDL receptor function, PCSK9 inhibitors may be effective to varying degrees [13,14].
Inclisiran
Inclisiran (Leqvio®) is a small interfering RNA (siRNA) that inhibits translation of the protein PCSK9 (Fig. 1) . Three trials enrolling patients with either heterozygous FH or pre-existing HKE evaluated the efficacy of this drug in addition to statins [15]. The primary endpoint in these studies was the reduction in LDL-C levels, and administration of the drug or placebo was subcutaneously at baseline and months 3, 9, and 15. Overall, the reduction in LDL-C levels was approximately 45%. To date, no study has documented a benefit for morbidity or mortality, but these are ongoing. Inclisiran was approved for marketing by the EMA in 2020 and by the FDA in 2021. Interestingly, the National Institute for Health and Care Excellence (NICE) in the UK endorsed its use in general practice in the UK even before any outcome studies were published. One of the advantages of Inclisiran is its pharmacokinetics, which results in a permanent reduction in LDL-C concentrations. Inclisiran is administered at 0 and three months and then twice annually, which ensures improved compliance (adherence). On the other hand, potential adverse events may persist, but its safety profile remains excellent to date. In all studies, includeisiran was used in conjunction with proven lipid-lowering treatment.
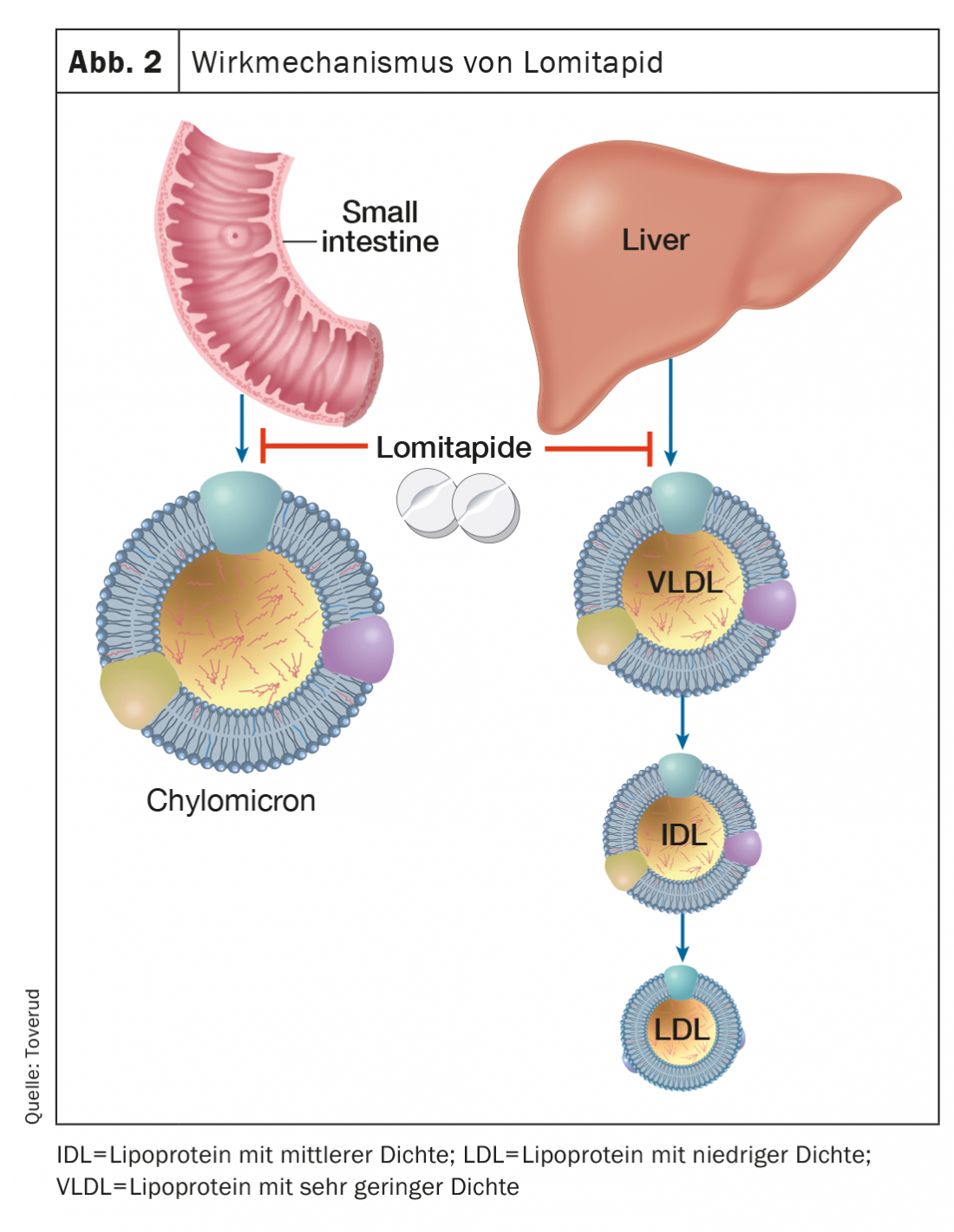
Lomitapide
Lomitapide, an inhibitor of the microsomal triglyceride transfer protein, inhibits the formation of apolipoprotein B, which contains lipoproteins in the intestine and liver, thereby lowering serum LDL-C, independent of LDL receptors (Fig. 2). In a dose-dependent manner, LDL-C concentrations can be reduced by approximately 50% [16]. Additional treatment with lomitapide may prolong the intervals between LDL apheresis in selected and motivated patients with homozygous FH. Side effects include gastrointestinal disturbances, elevated liver enzymes, and increased liver fat. Lomitapide (Lojuxta®) was approved in 2013 for the treatment of homozygous FH in adults. On the other hand, the costs are very high, which limits availability.
Bempedoic acid
Bempedoic acid is approved in the European Union (Nilemdo®) and in the United States (Nexletol®) for the treatment of hypercholesterolemia. As a prodrug, bempedoic acid requires activation by very long-chain acyl- CoA synthetase-1, which is expressed primarily in the liver (but not in skeletal muscle). The active metabolite inhibits adenosine triphosphate citrate lyase, an essential enzyme in the cholesterol synthesis pathway upstream of 3-hydroxy-3-methylglutaryl-CoA reductase (Fig. 3) . As with statins, inhibition of cholesterol synthesis leads to upregulation of LDL receptors and thus clearance of LDL particles. With a half-life of 15-24 hours, bempedoic acid can be administered orally once daily. As monotherapy, bempedoic acid reduced LDL-C concentrations by up to 25% (placebo-subtracted) in patients with baseline values of 3.4-5.7 mmol/l [17].
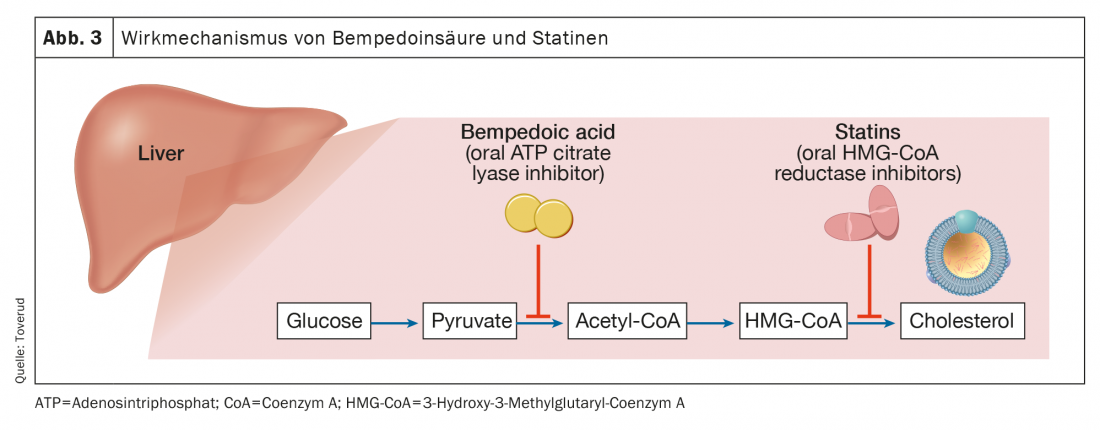
As mentioned earlier, the need has arisen to achieve greater reductions in LDL-C than those achieved with statins alone, and bempedoic acid may play a role. In patients receiving background statin therapy whose LDL-C levels remained between 3.0 and 5.7 mmol/l, bempedoic acid 180 mg daily further reduced LDL-C by ~20% despite targeting the same cholesterol biosynthesis pathway as statins but via different enzymes [18]. Rates of adverse events, including muscle-related symptoms, were similar to placebo [19]. Similarly, in patients with atherosclerotic cardiovascular disease or FH or both whose LDL-C levels remained >1.8 mmol/l with maximally tolerated lipid therapy (including statin), reductions in LDL-C of 17-18 were observed [20]. In a parallel study of similar patients with LDL-C levels >2.6 mmol/l at their first examination, a corresponding reduction was observed in which patients did not need to take a statin to participate in the study [21]. Common side effects of bempedoic acid include increased uric acid [20,21] and an increase in gout [21,22]. A trend toward worsening renal function was indicated in a recent meta-analysis [21].
In recent years, the need to manage lipids in patients who cannot tolerate statins has emerged. While randomized controlled trials show low rates of muscle symptoms, observational data indicate that up to one-third of statin-treated patients report absolute intolerance or inability to take adequate doses to achieve LDL-C goals [22]. In a substantial number of patients, symptoms that are considered side effects of statins are likely nocebo effects. Studies in patients with muscle-related symptoms during statin therapy show that most people are unable to distinguish periods of placebo therapy from periods of statin therapy [23,24]. Studies have been directed toward the use of bempedoic acid in patients with statin intolerance [25] and as add-on therapy to ezetimibe [26]. In patients with statin intolerance, LDL-C levels of 4.1 mmol/l, of which 21% were observed [25]. In a study of statin-intolerant patients taking ezetimibe, one-third of whom were taking a low-dose statin, LDL-C concentrations were reduced by >28% [27]. Bempedoic acid was well tolerated by these patient groups, with no increase in muscle symptoms.
Given the cost of PCSK9 inhibitors, follow-up studies have sought to achieve LDL-C concentrations as low as those provided by PCSK9 inhibitors. In a study of fixed-dose bempedoic acid plus ezetimibe, in patients at high cardiovascular risk with a mean baseline LDL-C of 3.9 mmol/l despite maximal statin therapy, reductions in LDL-C levels were substantial (>38%), although lower than those expected with a PCSK9 inhibitor [28]. Triple therapy with bempedoic acid, ezetimibe, and atorvastatin (20 mg/day) resulted in approximately equivalent LDL-C lowering of >64%, as would be expected with a PCSK9 inhibitor [29], although no head-to-head studies have been published. The addition of bempedoic acid alongside background therapy with once-monthly evolocumab 420 mg significantly reduced LDL-C by almost 30% [29].
In general, reductions in LDL-C levels with bempedoic acid appear to be somewhat greater in patient groups not taking a statin [18–20]. With regard to other lipid fractions, bempedoic acid reduces apolipoprotein B, triglycerides, and concentrations of non-high density lipoprotein cholesterol (non-HDL-C). Decreases in CRP concentrations observed in several studies may be promising but require further understanding of the mechanism [21,22,26].
The role for bempedoic acid going forward appears to be in (1) patients who are statin intolerant combined with other tolerated lipid-lowering agents and in (2) patients who require additional therapies to achieve LDL-C goals. The ongoing Cardiovascular Outcomes (CLEAR Outcome) study in patients with a history of, or at high risk of, HKE, statin intolerance, and LDL-C levels ≥2.6 mmol/l will further clarify the role of the drug and whether potential reductions in clinical endpoints relate exclusively to lipid lowering or also to a reduction in CRP.
Due to high costs, the availability of PCSK9 inhibitors is limited, as mentioned. With the advent of biosimilars and other forms of PCSK9 inhibition, prices may decrease in the future and this effective and well-tolerated therapy may become available to a larger number of patients.
Triglycerides/combined hyperlipidemia-directed therapies.
High triglycerides despite LDL-C-lowering therapies are one of the most common problems in clinical practice. Patients with hypertriglyceridemia may be at high residual risk for cardiovascular disease [30]. Given that lifestyle is emerging as a major cause of hypertriglyceridemia in patients with metabolic syndrome, abdominal obesity, or type 2 diabetes mellitus (T2DM), as well as in people who consume too much alcohol or are physically inactive, what more can a physician do to address these risk factors? While diet, activity and weight loss are cornerstones of treatment, genetics and other non-modifiable factors also play a role. For example, patients with familial combined hyperlipidemia are common in clinical practice and may have hypertriglyceridemia despite normal body weight [31]. A very rare condition is monogenic familial hyperchylomicronemia syndrome.
Epidemiological and genetic evidence has supported the hypothesis that variants of several key genes of triglyceride metabolism affecting triglycerides and triglyceride-rich and residual lipoproteins are causally associated with cardiovascular disease and overall mortality [32]. Through this evidence, new therapies have emerged that target these manifestations.
Eicosapentaenoic acid
Fish oil supplements have been touted for decades for their beneficial cardiovascular effects. They have also been used quite extensively in clinical practice to reduce triglyceride concentrations, but studies with omega-3 fatty acids have not demonstrated their protection with respect to HKE [33]. Therefore, the results of the REDUCE-IT study (Reduction of Cardioascular Events with Icosapent Ethyl-Interention Trial ) came as a surprise. Patients receiving 4 g of icosapent-ethyl or a placebo-containing mineral oil daily were shown to have a 25% reduction in the primary end point of a composite of cardiovascular death, nonfatal myocardial infarction (heart attack), nonfatal stroke, coronary revascularization, or unstable angina [34]. Further analysis showed consistent benefits for all patient subgroups and a 31% reduction in (first and subsequent) ischemic events [35]. The majority of patients included in the study were based on an existing HKE (71%); almost 60% had diabetes and the mean triglyceride level was 2.4 mmol/l. For example, the impressive reduction occurred in events in a group of patients at high cardiovascular risk, well over 90% of whom were treated with moderate- or high-intensity statins.
Since that publication, questions have focused on the difference between icosapent-ethyl, which was used in the REDUCE-IT study, and omega-3 carboxylic acid, which is a mixture of eicosapentaenoic acid (EPA) and docosahexaenoic acid. This compound was used at a dose of 4 g/day in the STRENGTH (Statin Residual Risk Reduction with Epanoa in High Cardioascular Risk Patients with Hypertriglyceridemia ) trial, a study that showed no reduction in cardiovascular events [36]. It has not yet been fully elucidated whether these differences can be attributed to possible damage from docosahexaenoic acid or to the higher dosage of EPA in the REDUCE-IT study compared with the STRENGTH study. Another explanation that has been proposed is due to the choice of placebo in the REDUCE-IT trial. In the mineral oil control group, LDL-C, apolipoprotein B, and C-reactive protein levels increased by 10.9%, 7.8%, and 32.3%, respectively, suggesting that the benefit of icosapent-ethyl may have been partially confounded by harm in the control group [34,37]. However, EPA may have properties that mitigate some of these differences.
The mechanisms of the benefits observed in the REDUCE-IT trial may be multifactorial, as the observed reductions in risk exceeded expectations for the degree of triglyceride reduction. On average, triglyceride levels decreased by 18.3% (-0.44 mmol/l) in the icosapent-ethyl group, while they increased by 2.2% in the placebo group. Because of triglyceride variation, approximately 10% of participants had normal levels, but risk reductions were consistent across all baseline triglyceride levels. Omega-3 fatty acids may reduce inflammation, influence cardiac arrhythmias due to modulation of membrane fluidity, and attenuate atherosclerotic plaque formation and progression [38]. On the one hand, although there was a significant 30% reduction in sudden cardiac deaths in the icosapent-ethyl group in the REDUCE-IT trial (a tertiary analysis), the rate of atrial fibrillation was significantly higher in the icosapent-ethyl group than in the placebo group (5.3% versus 3.9%) [34].
Despite some uncertainties, a review of icosapent-ethyl (Vazkepa®) by the EMA in 2021 for approval of the drug as a treatment to reduce the risk of cardiovascular events in high-risk statin-treated patients with elevated triglycerides (≥1.7 mmol/l) and existing HKE (or diabetes and one or more additional cardiovascular risk factors).
Pemafibrate
Fibrates are peroxisome proliferator-activated receptor (PPAR) agonists that have been used as triglyceride-lowering drugs for decades. Since most studies included a wide range of participants, their cardioprotective effects have been questioned and are mainly observed in subgroups with hypertriglyceridemia [39]. Pemafibrate stands out as a novel highly selective PPAR-α modulator that leads to significant reductions in triglyceride and residual cholesterol particles. However, a recent study found no significant reductions in non-HDL-C [40], suggesting that potential beneficial effects may be primarily related to reductions in triglyceride and triglyceride residues. The ongoing PROMINENT (Pemafibrate to Reduce Cardio ascular Outcomes by Reducing Triglycerides in Patients with Diabetes ) study has enrolled over 10 000 participants with T2DM (primary or secondary prevention), triglycerides of 2.26-5.64 mmol/l and HDL-C levels <1.03 mmol/l randomized to pemafibrate or placebo, with the study endpoint determined by events. The results will be presented shortly [41]. Positive results could significantly improve the treatment of patients with T2DM.
Apolipoprotein C-III and angiopoietin-like protein therapies.
Control of triglyceride metabolism involves a number of proteins and enzymatic pathways. Triglycerides in chylomicrons and very low-density lipoproteins undergo intravascular lipolysis by lipoprotein lipase to release free fatty acids that are used or stored as fuel. Central proteins regulating these processes are apolipoprotein C-III (ApoCIII), angiopoietin-like protein 3 (ANGPTL3), and ANGPTL4, all potent inhibitors of lipoprotein lipase (Fig 4).
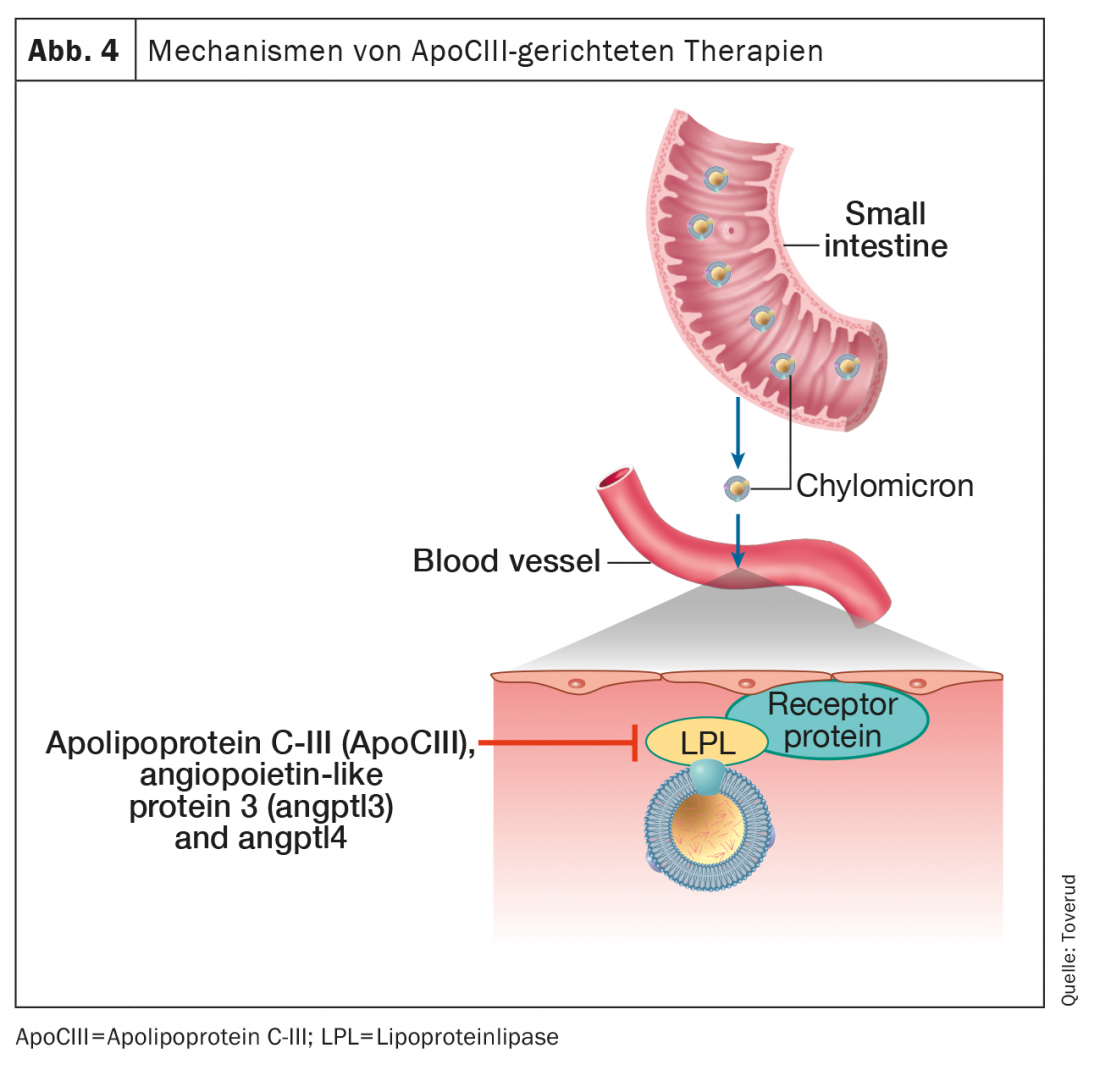
Gene evidence links loss-of-function variants of ApoCIII to reduced triglyceride levels and cardiovascular risk [42]. In addition, ApoCIII appears to contribute to the atherogenicity of several lipoproteins to which it is attached, including HDL [43]. Notably, the low risk of HKE in heterozygotes with apoCIII loss of function appears to be mediated by its association with low residual cholesterol rather than low LDL-C [44], further establishing the role of triglyceride residues in atherosclerosis. These insights from genetics and pathophysiology have acted as key catalysts for the development of therapeutic drugs against hypertriglyceridemia by inhibiting ApoCIII.
A recent study found that ANGPTL3 levels remained independent determinants of cardiovascular events, after adjustment for traditional risk factors and lipid-lowering medications [45].
Accordingly, loss-of-function mutations in the gene ANGPTL3 have been shown to be associated with hypobeta-lipoproteinemia and decreased triglyceride levels, and LDL-C and HDL-C levels, and risk of coronary artery disease [44], whereas mutations in the gene ANGPTL4 are associated with decreased triglycerides and increased HDL cholesterol levels [45]. Patients with ANGPTL3 deficiency did not show coronary atherosclerosis [46], leading to the conclusion that ANGPTL3 blockers may be promising risk-reducing agents. On the other hand, the development of angptl4 inhibitors was hampered by the observation of lipogranulomatous lesions in the intestine of angptl4-deficient mice.
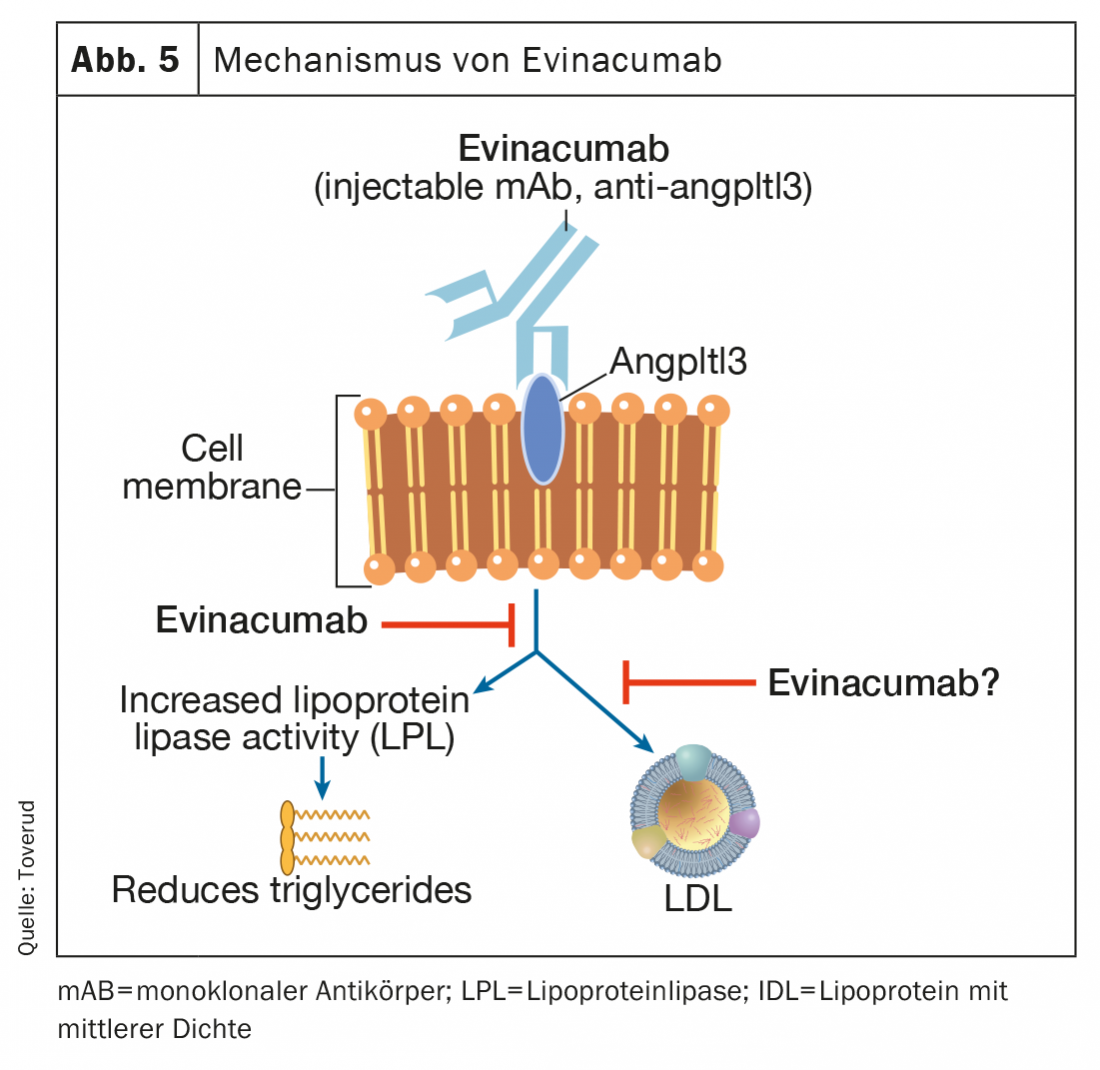
Evinacumab
Evinacumab is a fully human monoclonal antibody that binds to and inhibits angptl3 inhibitors, thereby increasing lipoprotein lipase activity (Fig. 5) . Evinacumab lowers elevated triglyceride levels, but not in patients with severely elevated triglycerides due to the lack of lipoprotein lipase activity in the rare familial hyperchylomicronemia syndrome [47,48]. Evinacumab also lowers LDL-C levels through a mechanism independent of LDL receptors that is not fully understood. In a recent phase 3 study in patients with homozygous FH, monthly intravenous infusions of evinacumab reduced LDL-C levels by 47% (49% versus placebo) and appeared to be a promising new treatment option for these difficult-to-treat patients [49]. Flu-like symptoms occurred more frequently in patients receiving evinacumab. Long-term adverse events in atherosclerosis and pregnancy are recorded from an ongoing user registry. Evinacumab (Evkeeza®) was approved in 2021 for the treatment of homozygous FH aged 12 years and older.
Vupanorsen
Vupanorsen is an antisense oligonucleotide at ANGPTL3 mRNA being developed as a potential treatment for dyslipidemia [50]. In patients with diabetes, hepatic steatosis (fatty liver), and hypertriglyceridemia, the drug significantly reduced triglycerides and total and non-HDL cholesterol without reducing platelet counts [51].
Volanesorsen
Volanesorsen is an antisense oligonucleotide against Apo-CIII mRNA that strongly reduces triglyceride and ApoCIII levels through pathways independent of lipoprotein lipase (Fig. 4). Randomized, controlled clinical trials have shown a reduction in triglyceride levels of approximately 70% and a reduced risk of acute pancreatitis associated with hypertriglyceridemia [52]. A significant step forward in the treatment of rare diseases has been made in patients with familial chylomicronemia syndrome, a rare and potentially fatal genetic disorder due to loss of lipoprotein lipase activity characterized by chylomicronemia with recurrent pancreatitis and few therapeutic options. Volanesorsen lowered triglyceride levels by 77%, and most participants achieved triglyceride levels below 8.5 mmol, a threshold associated with a significantly reduced risk of pancreatitis [53]. Volansorsen lowered apolipoprotein B-48 by 76% but increased LDL-C by 136% and total apolipoprotein B by 20%. Although these results may reflect a possible increased cardiovascular risk, levels of atherogenic lipoproteins were very low, as typically seen in patients with familial chylomicronemia.
A major adverse effect of volanesorsen is thrombocytopenia, which led the FDA to withhold approval, as well as concerns about major bleeding. However, thrombocytopenia is reversed by stopping the drug. The EMA approved volanesorsen (Waylivra®) in adult patients with familial chylomicronemia syndrome in 2019.
Olezarsen
Olezarsen is an antisense oligonucleotide that targets hepatic ApoCIII mRNA to inhibit ApoCIII protein production (Fig. 4). In a recent study, treatment with olezarsen for 6-12 months dose-dependently lowered triglycerides by 23-60, with no changes in platelet count, liver, or renal function, in participants with elevated triglycerides and high cardiovascular risk or cardiovascular disease [51].
Therapies against elevated lipoprotein (A)
The link between elevated Lp(a) and cardiovascular disease was established by studies after the molecule was identified in 1968 by Berg in Oslo, Norway. A meta-analysis of prospective studies found an approximate increase in coronary heart disease risk of 1.16 (1.11-1.22) per 3.5-fold increase in Lp(a) concentration, decreasing only slightly to 1.13 (1.09-1.18) after adjustment [54].
Associations have also been demonstrated between Lp(a) and mortality, as well as stroke, peripheral artery disease, and calcified aortic valve stenosis. This brief review targets specific therapies for apo(a) only. It is believed that although some studies have found that Lp(a) lowering by 20% to 25% did not represent a reduced cardiovascular risk beyond concomitant LDL-C lowering, this observation may be explained by the assumption that large reductions in absolute levels are required, as studies of the following novel therapies have found [55].
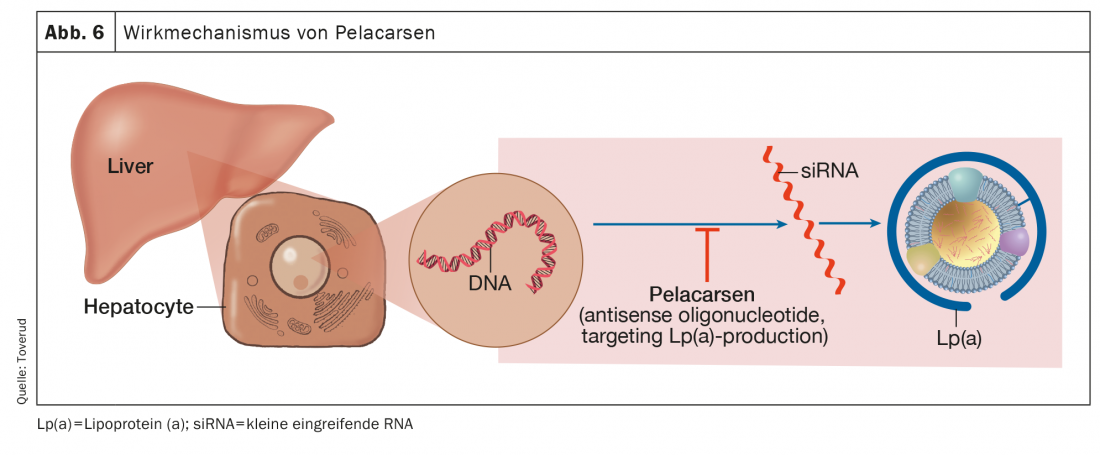
Pelacarsen
Pelacarsen is the antisense oligonucleotide targeting hepatic apo(a) production that has made the most progress in studies that have been completed or initiated (Fig. 6). Compared with placebo, pelacarsen dose-dependently and consistently reduced Lp(a) concentrations by up to 80% in patients with elevated levels and existing HKE [56].
In addition, decreases in oxidized phospholipids were observed on apolipoprotein B and apo(a), components of Lp(a) predicted to have pro-inflam-ma–to-ric effects. The Lp(a) HORIZON study is a randomized, double-blind, placebo-controlled multicenter trial to assess the effect of pelacarsen on cardiovascular events in patients with existing HKE and LP(a) concentrations ≥175 nmol/l [57].
Small interventional RNA therapy
Olpasiran, a small intervening molecule designed to directly inhibit Lp(a) messenger RNA, showed large, dose-dependent, and long-lasting reductions of 71% to 97% in Lp(a) concentrations with effects lasting several months [58]. Recently, the results of an escalation study of another small intervening RNA therapy (SLN360) that inhibits translation of the apo(A) coding gene in hepatocytes and was also administered as a single dose were reported. Average Lp(a) reductions of 98% were observed in the group receiving the highest dose after up to 150 days [59]. Safety issues related to these two therapies will require longer and larger studies.
Conclusion
Taken together, there is an unmet need for more effective lipid-lowering therapies as well as an expansion of the current therapeutic armamentarium. Recent advances in pharmacotherapy suggest a number of potential mechanistic and pharmacologic pathways that are being used with the goal of bringing more patients to lipid targets. The field is advancing more rapidly than in recent decades, and there may be significant changes in the way we address the global atherosclerotic burden in the future.
Source: This paper is a secondary publication, a translation from the original English-language article published in the journal Kardiol.Pol. published. Reference is: cardiol.pol 2022; 80(7-8): 741-749. doi: 10.33963/KP.a2022.0117.
We thank Kari C. Toverud (Certified Medical Illustrator) for the figures.
Take-Home Messages
- Atherosclerosis remains the leading cause of death worldwide.
- Much of the onset and development of atherosclerosis is caused by dyslipidemia.
- With the advent of statins, ezetimibe, and most recently the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, physicians in all specialties have access to a therapeutic arsenal to address this important pathophysiological driver.
- Nevertheless, there remains a great unmet need for optimization strategies for pharmacotherapeutic lipid lowering.
- These focus on reducing triglycerides (TG) or lipoprotein(a) [Lp(a)].
Literature:
- WHO: Health topics in cardiovascular diseases 2021. Available online: www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (Accessed April 28, 2022).
- Atar D, Jukema JW, Molemans B, et al: New cardiovascular prevention guidelines: how to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis 2021; 319: 51-61, doi: 10.1016/j.atherosclerosis.2020.12.013, indexed in Pubmed: 33476944.
- Sverre E, Peersen K, Husebye E, et al: Unfavourable risk factor control after coronary events in routine clinical practice. BMC Cardiovasc Disord 2017; 17(1): 40, doi: 10.1186/s12872-016-0387-z, indexed in Pubmed: 28109259.
- Setny M, Jankowski P, Kamiński K, et al: Secondary prevention of coronary heart disease in Poland: does sex matter? Results from the POLASPIRE survey. Pol Arch Intern Med 2022; 132(3), doi: 10.20452/pamw.16179, indexed in Pubmed: 34935325.
- Mundal L, Igland J, Ose L, et al: Cardiovascular disease mortality in patients with genetically verified familial hypercholesterolemia in Norway during 1992-2013. Eur J Prev Cardiol 2017; 24(2): 137-144, doi: 10.1177/2047487316676135, indexed in Pubmed: 27794106.
- Bogsrud MP, Græsdal A, Johansen D, et al: LDL-cholesterol goal attainment, cardiovascular disease, and attributed risk of Lp(a) in a large cohort of genetically predominantly verified familial hypercholesterolemia. J Clin Lipidol. 2019; 13(2): 279-286, doi: 10.1016/j.jacl.2019.01.010, indexed in Pubmed: 30910667.
- Mach F, Baigent C, Catapano AL, et al: ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020; 41(1): 111-188, doi: 10.1093/eurheartj/ehz455, indexed in Pubmed: 31504418.
- O’Donoghue ML, Giugliano RP, Atar D, et al: Long-Term Evolocumab in Patients With Established Atherosclerotic Cardiovascular Disease. Circulation 2022 Oct 11; 146(15): 1109-1119. doi: 10.1093/eurheartj/ehz430, indexed in Pubmed: 31270529.
- Kastelein JJP, Ginsberg HN, Langslet G, et al: ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozy- gous familial hypercholesterolaemia. Eur Heart J 2015; 36(43): 2996-3003, doi: 10.1093/eurheartj/ehv370, indexed in Pubmed: 26330422.
- Raal FJ, Stein EA, Dufour R, et al: RUTHERFORD-2 Investigators. PCSK9 in- hibition with evolocumab (AMG 145) in heterozygous familial hyper- cholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 385(9965): 331-340, doi: 10.1016/S0140-6736(14)61399-4, indexed in Pubmed: 25282519.
- Farnier M, Hovingh GK, Langslet G, et al: Long-term safety and efficacy of alirocumab in patients with heterozygous familial hypercholesterolemia: An open-label extension of the ODYSSEY program. Atherosclerosis 2018; 278: 307-314, doi: 10.1016/j.atherosclerosis.2018.08.036, indexed in Pubmed: 30293878.
- Koren MJ, Sabatine MS, Giugliano RP, et al: Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol 2019; 74(17): 2132-2146, doi: 10.1016/j.jacc.2019.08.1024, indexed in Pubmed: 31648705.
- Santos RD, Stein EA, Hovingh GK, et al: Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol 2020; 75(6): 565-574, doi: 10.1016/j.jacc.2019.12.020, indexed in Pubmed: 32057369.
- Blom DJ, Harada-Shiba M, Rubba P, et al: Efficacy and safety of alirocumab in adults with homozygous familial hypercholesterolemia: the ODYS- SEY HoFH trial. J Am Coll Cardiol 2020; 76(2): 131-142, doi: 10.1016/j. jacc.2020.05.027, indexed in Pubmed: 32646561.
- Santulli G, Jankauskas SS, Gambardella J: Inclisiran: a new milestone on the PCSK9 road to tackle cardiovascular risk. Eur Heart J Cardiovasc Pharmacother 2021; 7(3): e11-e12, doi: 10.1093/ehjcvp/pvab014, indexed in Pubmed: 33655296.
- Cuchel M, Bloedon LT, Szapary PO, et al: Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med 2007; 356(2): 148-156, doi: 10.1056/NEJMoa061189, indexed in Pubmed: 17215532.
- Ballantyne CM, Davidson MH, Macdougall DE, et al: Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol 2013; 62(13): 1154-1162, doi: 10.1016/j.jacc.2013.05.050, indexed in Pubmed: 23770179.
- Ballantyne CM, McKenney JM, MacDougall DE, et al: Effect of ETC-1002 on serum low-density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol 2016; 117(12): 1928-1933, doi: 10.1016/j.amjcard.2016.03.043, indexed in Pubmed: 27138185.
- Ray KK, Bays HE, Catapano AL, et al: CLEAR Harmony Trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019; 380(11): 1022-1032, doi: 10.1056/NEJMoa1803917, indexed in Pubmed: 30865796.
- Goldberg AC, Leiter LA, Stroes ESG, et al: Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA 2019; 322(18): 1780-1788, doi: 10.1001/jama.2019.16585, indexed in Pubmed: 31714986.
- Lin Y, Parco C, Karathanos A, et al: Clinical efficacy and safety outcomes of bempedoic acid for LDL-C lowering therapy in patients at high cardiovascular risk: a systematic review and meta-analysis. BMJ Open. 2022; 12(2): e048893, doi: 10.1136/bmjopen-2021-048893, indexed in Pubmed: 35210334.
- Bytyçi I, Penson PE, Mikhailidis DP, et al: Prevalence of statin intolerance: a meta-analysis. Eur Heart J 2022 [Epub ahead of print]: ehac015, doi: 10.1093/eurheartj/ehac015, indexed in Pubmed: 35169843.
- Wood FA, Howard JP, Finegold JA, et al: N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med 2020; 383(22): 2182-2184, doi: 10.1056/NEJMc2031173, indexed in Pubmed: 33196154.
- Herrett E, Williamson E, Brack K, et al: StatinWISE Trial Group. Statin treat- ment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ. 2021; 372: n135, doi: 10.1136/bmj.n135, indexed in Pubmed: 33627334.
- Laufs U, Banach M, Mancini GB, et al: Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019; 8(7): e011662, doi: 10.1161/JAHA.118.011662, indexed in Pubmed: 30922146.
- Ballantyne CM, Banach M, Mancini GB, et al: Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: A randomized, placebo-controlled study. Atherosclerosis 2018; 277: 195-203, doi: 10.1016/j.atherosclerosis.2018.06.002, indexed in Pubmed: 29910030.
- Ballantyne CM, Laufs U, Ray KK, et al: Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020; 27(6): 593-603, doi: 10.1177/2047487319864671, indexed in Pubmed: 31357887.
- Rubino J, MacDougall DE, Sterling LR, et al: Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: A randomized clinical trial. Atherosclerosis 2021; 320: 122-128, doi: 10.1016/j.atherosclerosis.2020.12.023, indexed in Pubmed: 33514449.
- McKenney J, MacDougall D, Sterling L, et al: Lipid lowering with bempe- doic acid added to proprotein convertase subtilisin/kexin type 9 inhibitor therapy: a randomized controlled trial. J Clin Lipidol 2019; 13(3): e55-e56, doi: 10.1016/j.jacl.2019.04.092.
- Soehnlein O, Libby P. Targeting inflammation in atherosclerosis – from experimental insights to the clinic. Nature Rev 2021; 20(8): 589-610, doi: 10.1038/s41573-021-00198-1, indexed in Pubmed: 33976384.
- Ginsberg HN, Packard CJ, Chapman MJ, et al: Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J 2021; 42(47): 4791-4806, doi: 10.1093/eurheartj/ehab551, indexed in Pubmed: 34472586.
- Bello-Chavolla OY, Kuri-García A, Ríos-Ríos M, et al: Familial combined hyperlipidemia: current knowledge, perspectives, and controversies. Rev In- vest Clin. 2018; 70(5): 224-236, doi: 10.1007/springerreference_35144, indexed in Pubmed: 30307446.
- Aung T, Halsey J, Kromhout D, et al: Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol 2018; 3(3): 225-234, doi: 10.1001/jamacardio.2017.5205, indexed in Pubmed: 29387889.
- Bhatt DL, Steg PG, Miller M, et al: REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019; 380(1): 11-22, doi: 10.1056/NEJMoa1812792, indexed in Pubmed: 30415628.
- Peterson BE, Bhatt DL, Steg PhG, et al: REDUCE-IT Investigators, RE- DUCE-IT Investigators, REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019; 73(22): 2791-2802, doi: 10.1016/j.jacc.2019.02.032, indexed in Pubmed: 30898607.
- Nicholls SJ, Lincoff AM, Garcia M, et al: Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020; 324(22): 2268-2280, doi: 10.1001/jama.2020.22258, indexed in Pubmed: 33190147.
- Doi T, Langsted A, Nordestgaard BG: A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs. Eur Heart J 2021; 42(47): 4807-4817, doi: 10.1093/eu-rheartj/ehab555, indexed in Pubmed: 34455435.
- Mason RP, Libby P, Bhatt DL: Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterio- scler Thromb Vasc Biol 2020; 40(5): 1135-1147, doi: 10.1161/ATVBA-HA.119.313286, indexed in Pubmed: 32212849.
- Liu ZL, Li GQ, Bensoussan A, et al: Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375(9729): 1875-1884, doi: 10.1016/S0140-6736(10)60656-3, indexed in Pubmed: 20462635.
- Ginsberg HN, Hounslow NJ, Senko Y, et al: Efficacy and safety of K-877 (pe-mafibrate), a selective pparα modulator, in european patients on statin therapy. Diabetes Care 2022; 45(4): 898-908, doi: 10.2337/dc21-1288, indexed in Pubmed: 35238894.
- Pradhan AD, Paynter NP, Everett BM, et al: Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J 2018; 206: 80-93, doi: 10.1016/j.ahj.2018.09.011, indexed in Pubmed: 30342298.
- Dib I, Khalil A, Chouaib R, et al: Apolipoprotein C-III and cardiovascular diseases: when genetics meet molecular pathologies. Mol Biol Rep 2021; 48(1): 875-886, doi: 10.1007/s11033-020-06071-5, indexed in Pubmed: 33389539.
- Wulff AB, Nordestgaard BG, Tybjærg-Hansen A. APOC3 loss-of-function mutations, remnant cholesterol, low-density lipoprotein cholesterol, and cardiovascular risk: mediation- and meta-analyses of 137 895 individuals. Arterioscler Thromb Vasc Biol 2018; 38(3): 660-668, doi: 10.1161/AT- VBAHA.117.310473, indexed in Pubmed: 29348120.
- Stitziel NO, Khera AV, Wang X, et al: PROMIS and Myocardial Infarction Genetics Consortium Investigators. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 2017; 69(16): 2054-2063, doi: 10.1016/j.jacc.2017.02.030, indexed in Pubmed: 28385496.
- Dewey FE, Gusarova V, O’Dushlaine C, et al: Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 2016; 374(12): 1123-1133, doi: 10.1056/NEJMoa1510926, indexed in Pubmed: 26933753.
- Hussain A, Sun C, Selvin E, et al: Triglyceride-rich lipoproteins, apolipo- protein C-III, angiopoietin-like protein 3, and cardiovascular events in older adults: Atherosclerosis Risk in Communities (ARIC) study. Eur J Prev Cardiol 2022; 29(2): e53-e64, doi: 10.1093/eurjpc/zwaa152, indexed in Pubmed: 33580780.
- Ahmad Z, Banerjee P, Hamon S, et al: Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertri- glyceridemia. Circulation 2019; 140(6): 470-486, doi: 10.1161/CIRCULATIONAHA.118.039107, indexed in Pubmed: 31242752.
- Ahmad Z, Pordy R, Rader D, et al: Inhibition of angiopoietin-like protein 3 with evinacumab in subjects with high and severe hypertriglyceridemia. J Am Coll Cardiol 2021; 78(2): 193-195, doi: 10.1016/j.jacc.2021.04.091, indexed in Pubmed: 34238441.
- Raal FJ, Rosenson RS, Reeskamp LF, et al: ELIPSE HoFH Investiga- tors. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 2020; 383(8): 711-720, doi: 10.1056/NEJMoa2004215, indexed in Pubmed: 32813947.
- Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, et al: Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with dia- betes, hepatic steatosis and hypertriglyceridaemia. Eur Heart J 2020; 41(40): 3936-3945, doi: 10.1093/eurheartj/ehaa689, indexed in Pubmed: 32860031.
- Tardif JC, Karwatowska-Prokopczuk E, Amour ES, et al: Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk. Eur Heart J. 2022; 43(14): 1401-1412, doi: 10.1093/eu- rheartj/ehab820, indexed in Pubmed: 35025993.
- Gelrud A, Digenio A, Alexander V, et al: Treatment with volanesorsen reduced triglycerides and pancreatititis in patients with FCS and sHTG vs placebo: results of the APPROACH and COMPASS studies. Atherosclerosis; 32(Suppl 2018): 157-158.
- Witztum JL, Gaudet D, Freedman SD, et al: Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019; 381(6): 531-542, doi: 10.1056/nejmoa1715944, indexed in Pubmed: 31390500.
- Erqou S, Kaptoge S, Perry PL, et al: Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009; 302(4): 412-423, doi: 10.1001/jama.2009.1063, indexed in Pubmed: 19622820.
- Ference BA: The potential clinical benefit of lowering lipoprotein(a). JAMA 2022 [Epub ahead of print], doi: 10.1001/jama.2022.5333, indexed in Pubmed: 35368050.
- Tsimikas S, Karwatowska-Prokopczuk E, Xia S, et al: AKCEA-APO(a)-LRx Study Investigators. Lipoprotein (a) reduction in persons with cardiovascular disease. N Engl J Med 2020; 382(3): 244-255, doi: 10.1056/NEJ-Moa1905239, indexed in Pubmed: 31893580.
- Assessing the Impact of Lipoprotein (a) Lowering With Pelacarsen (TQJ230) on Major Cardiovascular Events in Patients With CVD. https://clinicaltrials.gov/ct2/show/NCT04023552 (April 28, 2022).
- Koren MJ, Moriarty PM, Baum SJ, et al: Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat Med 2022; 28(1): 96-103, doi: 10.1038/s41591-021-01634-w, indexed in Pubmed: 35027752.
- Nissen SE, Wolski K, Balog C, et al: Single ascending dose study of a short interfering RNA targeting lipoprotein (a) production in individuals with elevated plasma lipoprotein(a) levels. JAMA 2022 [Epub ahead of print], doi: 10.1001/jama.2022.5050, indexed in Pubmed: 35368052.
CARDIOVASC 2022; 21(4): 6-15











