Neutropenia is a frequent guest in the practice as an independent clinical picture, but also as an accompanying symptom of many diseases and as an adverse drug reaction. The accompanying susceptibility to infection can be a challenge in many cases. Good management prevents dangerous consequences.
Neutropenia is a frequent guest in hematological and oncological practice as an independent clinical picture, but also as an accompanying symptom of many diseases and as an adverse drug reaction. The accompanying susceptibility to infection can be a challenge in many cases. This is due in part to the breadth of the clinical spectrum. From the emergency situation of febrile neutropenia to the often harmless chronic form, everything is represented. Accordingly, for optimal management, it is important to know the cause of neutropenia.
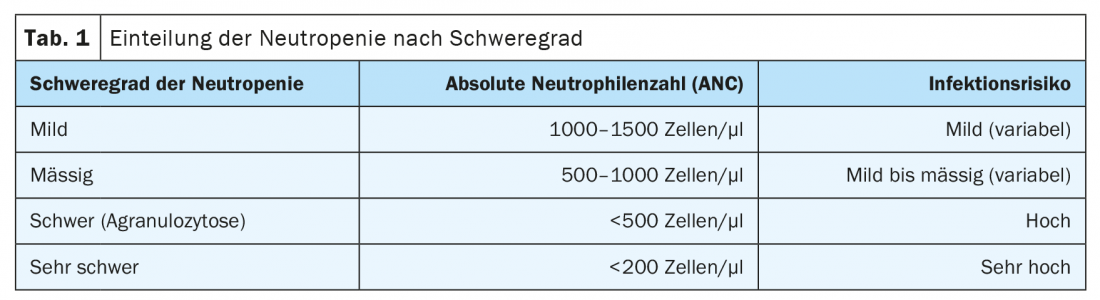
Severity of neutropenia
Depending on the severity and thus the risk of infection, the therapeutic approach also differs. A mild form is understood to have absolute neutrophil counts (ANC) between 1000 and 1500 cells per microliter. Moderate-grade neutropenia is present with ANC between 500 and 1000 cells per microliter, and severe neutropenia/agranulocytosis has ANC less than 500 cells per microliter (Table 1) .
While mild forms often do not require treatment, immediate, empiric use of broad-spectrum antibiotics and, if necessary, granulocyte colony-stimulating factor (G-CSF) is indicated in cases of fever and agranulocytosis [1–3].
Not all neutropenia is the same
For the characterization of neutropenia, not only its severity is important, but also its course. Thus, a basic distinction is made between acute and chronic forms. The temporal dynamics, previous medications and infections experienced, as well as family history, can provide information about the underlying mechanism. There are primary and secondary forms of neutropenia (overview 1) . Primary neutropenia is much rarer than extrinsic neutropenia. However, they must be considered, especially in younger patients, chronic courses since childhood, and a positive family history [2–4].

Chronic idiopathic neutropenia is one of the most common primary neutropenias. Predominantly affected are women. With a duration of at least three months, it is a diagnosis of exclusion. Accordingly, there must be no genetic, infectious, inflammatory, autoimmune, malignant or drug-related cause. Although there is an increased susceptibility to bacterial infections, the disease usually has a benign course. Treatment with G-CSF is not always necessary and is not recommended for ANC levels above 500 cells/µl and without the presence of recurrent infections [5]. It must be weighed on an individual basis. So far, no cause has been found for the large individual differences in susceptibility to infection.
Benign ethnic neutropenia, which mainly affects people of African and Mediterranean origin, also usually has no clinical effects, or a mild course [6]. This is equally true for benign familial neutropenia, which is clearly genetic but independent of ethnicity [2]. While the genetic basis of benign familial neutropenia is unknown, benign ethnic neutropenia is associated with variants of the Duffy antigen receptor [7,8].
Another example of usually mild congenital neutropenia is cyclic neutropenia. This is an autosomal dominant inherited disorder, often with mutations in the ELANE gene [9]. It is characterized by periodic fluctuations in neutrophil counts with an oscillation period of about 21 days. During nadir, some patients develop oral aphthae or other infections, but the overall course is mostly benign [2].
Kostmann syndrome, on the other hand, also known as severe congenital neutropenia (SCN), is a condition that leads to high susceptibility to infection in childhood and increases the risk of developing acute myeloid leukemia [10]. Persistent neutropenia in children, adolescents, or young adults may indicate underlying bone marrow failure. In such cases, further clarification by specialized experts is recommended.
Although congenital and idiopathic neutropenias should not be neglected in the clarification of causes, isolated neutropenia is acquired in most cases. Various drugs, bone marrow diseases, deficiencies, infections and immunological processes can be considered as triggers.
A common phenomenon: drug-induced neutropenia
The largest proportion of neutropenias is due to medications [1,11–13]. The incidence of iatrogenic agranulocytosis is about 1-5 per million population annually, with a mortality rate of about 5% [1,14]. Typically, there is a temporal association with drug therapy. In principle, many drugs can cause neutropenia by two different mechanisms, with more than half of the cases triggered by a few agents (review 2) [11–16,22]. On the one hand, drug-induced antibodies may lead to immune-mediated destruction of neutrophil granulocytes. On the other hand, agents with direct cytotoxic effects on myeloid precursors exist, such as phenothiazines, clozapine, dapsone, and procainamide [15].

Unlike immune-mediated neutropenia, direct toxicity is dose-dependent. While here the quantity makes the poison, the damage to granulocytes in the context of drug-induced antibody formation is independent of time and dose. Since sensitization is likely to last a lifetime, a small dose of the triggering substance is sufficient to cause recurrent neutropenia or even agranulocytosis. Due to the different mechanisms of neutrophil injury, the effects of different drugs are difficult to predict and characterize. Figure 1 gives an overview of some empirical values.
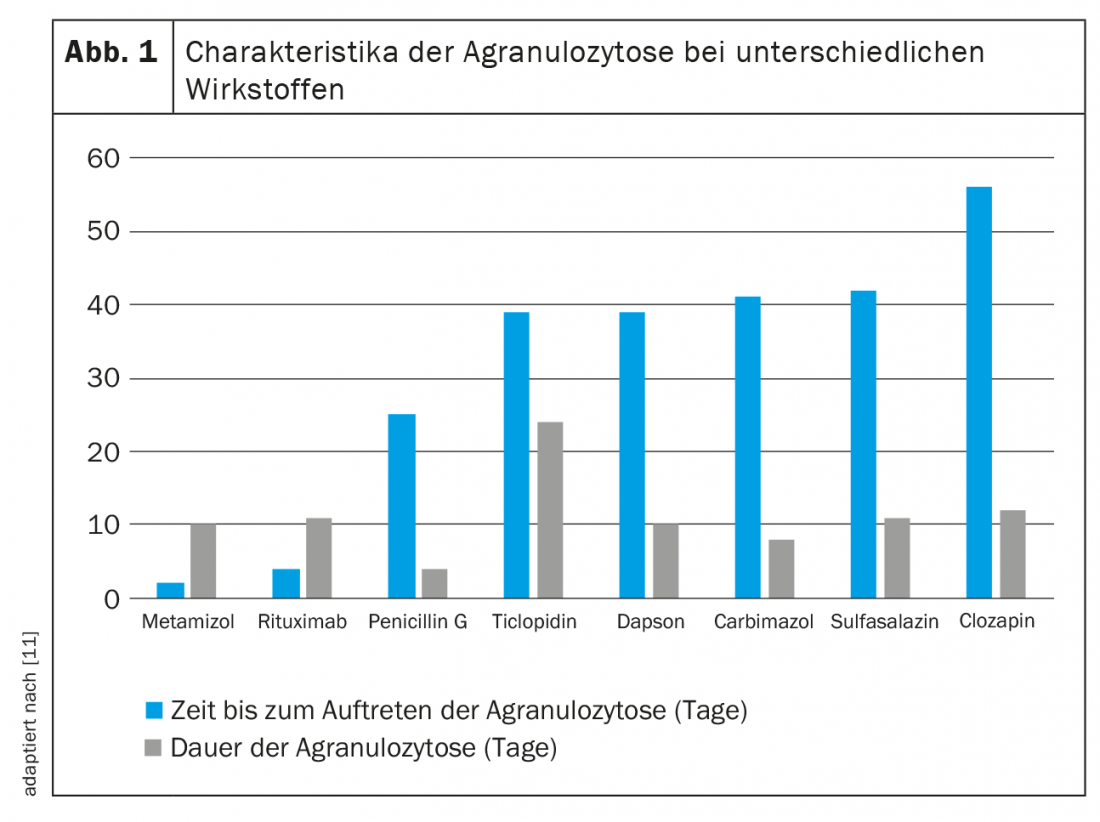
Probably the most notorious agent posing a risk of agranulocytosis is metamizole (Novalgin®). Corresponding data were published as early as 1964 [16]. Usually, neutropenia occurs in the first two months of treatment and normalizes within two weeks [11]. It is estimated that the risk of metamizole-induced neutropenia is between 1/116 and 1/466 000 exposures. The absolute risk is low for normal dosing and short use, with large geographic differences [17,18]. While the incidence is high in Sweden, for example, it appears to be lower in the Netherlands or Spain. This could be due to different patterns of use, as the risk increases with longer drug administration and unfavorable co-medication. The assumption that, for example, British, Irish, and Scandinavians have a higher risk of metamizole-related agranulocytosis because of their ethnicity was investigated in an epidemiological study [19]. This concluded that certain HLA alleles may also have an association with the development of neutropenia with metamizole therapy.
The bottom line is that, although critical from a hematologic perspective, metamizole is a defensible choice in a few cases despite the risk of agranulocytosis, especially compared with alternative analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs). These tend to have an even less favorable side effect profile, which should not be disregarded [20]. However, metamizole should be used cautiously and with due care. Although regular laboratory checks are not recommended in asymptomatic patients, affected individuals should be educated about the risk of agranulocytosis and its symptoms [21].
In general, advanced age, poor performance status, sepsis or shock, renal failure, and ANC values below 100 cells/µl are considered prognostically unfavorable factors in drug-induced neutropenia [1]. In contrast, therapy with intravenous broad-spectrum antibiotics and G-CSF improves prognosis [1]. With increasing experience in the management of affected patients, great progress has been made in this field in recent years. However, the administration of G-CSF in particular remains controversial. Statistically, administration shows shorter hospitalizations and less antibiotic use, but no clear guidelines exist regarding the use of G-CSF in non-chemotherapy, drug-induced agranulocytosis [13,22–24]. Hematopoietic growth factors should be given if neutrophil counts are less than 100 cells/µl; otherwise, the decision should be weighed on an individual basis.
Neutropenia and infections: The hen and the egg
Viruses such as HIV, EBV, CMV , hepatitis A, and Sars-CoV-2 can cause neutropenia. In addition, neutropenia can occur in typhoid fever, brucellosis, tularemia, shigellosis, and tuberculosis, with neutrophil deficiency again favoring infection. Rickettsiae and various parasites can also cause neutropenia. Influenza is more widespread in our latitudes and affects Switzerland every year. Mild and transient cases of neutropenia usually occur, but a clear association exists. The risk appears to be higher for influenza B than for influenza A [25]. Another risk that can be eliminated by the flu shot.
Management in everyday clinical practice
While fever in neutropenia is always an indication for adequate antibiotic therapy with or without G-CSF administration, the subsequent search for the cause often proves to be a lengthy process (Fig. 2) [2,3]. Chronic, especially mild, courses in adulthood are more suggestive of primary forms of neutropenia such as benign ethnic neutropenia. Acute onset, on the other hand, is indicative of a drug-induced or infectious genesis of the disorder, especially if there is a history of appropriate exposure. If the neutrophil counts normalize in the course of the disease, no further clarification is necessary in this case. However, if the etiology remains unclear, bone marrow examination should be performed.
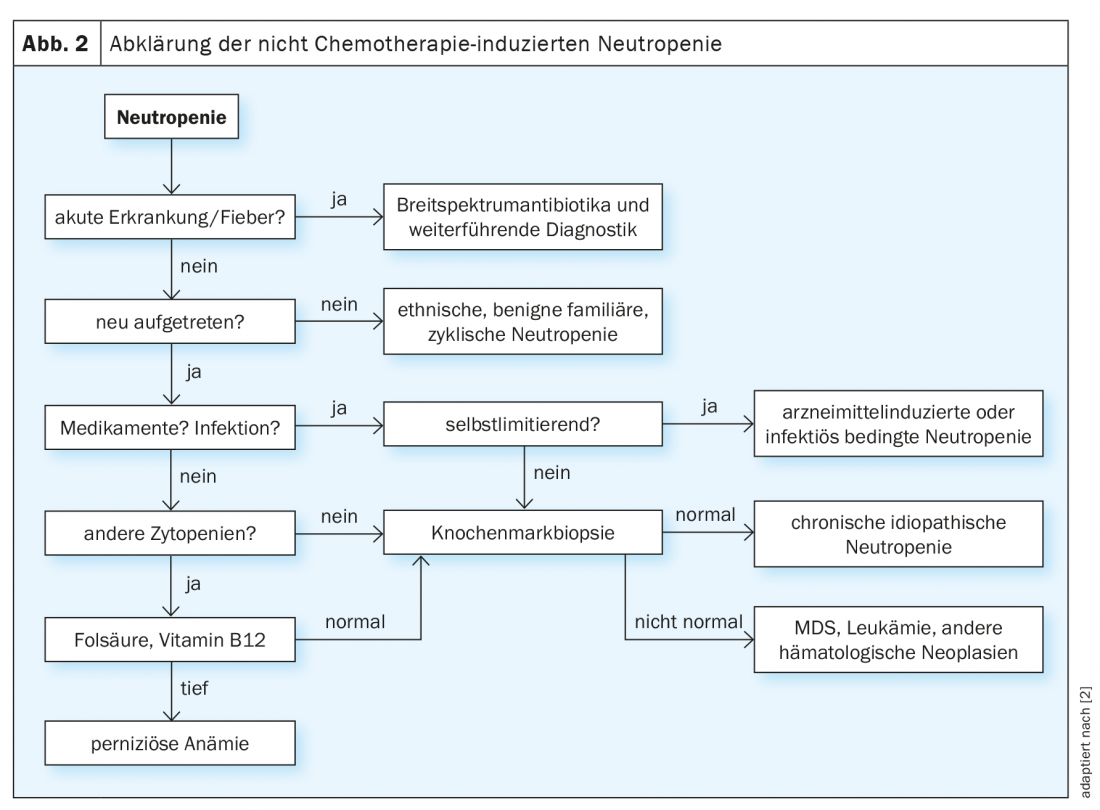
Treatment of nonchemotherapy-related neutropenia is primarily based on the individual risk of infection and cause, ranging from continuous G-CSF administration to a wait-and-see procedure. It is important that all patients know their individual risk of infection, as well as warning signs and precautions, and can act accordingly.
In case of acute infections, G-CSF administration may be considered [2,3,26]. This should never be based purely on neutrophil count and thus should only be used in symptomatic neutropenia. Even an increase in ANC above 250 to 300 cells/µl can dramatically reduce the rate of infection in symptomatic patients [2].
Of course, if available, the causative therapy of neutropenia is the first priority. Folic acid and vitamin B12 substitution as well as, for example, the treatment of autoimmune neutropenia with methotrexate or cyclophosphamide are just a few examples that illustrate the importance of a thorough investigation of the causes [27].
Special case COVID-19
There are only a few data on the administration of G-CSF in the presence of Sars-CoV-2 infection, but they are nevertheless rather worrying, especially in tumor patients. Thus, in a study that included 55 patients, a worse disease outcome was observed with treatment with G-CSF [28]. Those 16 individuals who received G-CSF had greater oxygen demand and mortality. A case study of three cases postulated favoring of inflammation and macrophage activation by G-CSF as a mechanism. In all patients, clinical deterioration occurred around 72 hours after drug administration [29]. Comparable observations were described in another case report of a 47-year-old COVID-19 patient [30].
The risk of treatment with G-CSF in the presence of COVID-19 can certainly not be conclusively assessed based on the current data. The need for additional data is great and this could lead the way for years to come.
Outlook
A retrospective analysis of all cases with isolated neutropenia below 500 cells/µl between 2015 and September 2020 is currently ongoing at the University Inselspital Bern. Patients undergoing chemotherapy or radiotherapy and those with concomitant other cytopenias were not included. The primary objective is to collect data on cause, therapy, infection and hospitalization rates, and other outcomes. We hope for the discovery of correlations that could be relevant for risk stratification and thus therapeutically.
Take-Home Messages
- Neutropenia/agranulocytosis should receive our attention because of the increased risk of infection with possible severe courses.
- Drug-induced neutropenia is the most common form of isolated neutropenia.
- The chronic benign forms of neutropenia in adults are diagnoses of exclusion and usually have a benign course.
- Treatment with G-CSF must always be considered on an individual basis and is recommended for recurrent infections and neutrophil counts below 0.5 ×109/L. Prophylactic antibiotic administration is not recommended.
- Neutropenia may occur as a result of COVID-19 infection. The effect of G-CSF in COVID-19 patients is still unclear. The use of G-CSF, especially in tumor patients, should be done with caution until further data are available.
Literature:
- Andres E, et al: Clinical presentation and management of drug-induced agranulocytosis. Expert review of hematology 2011; 4(2): 143-151.
- Gibson C, Berliner N: How we evaluate and treat neutropenia in adults. Blood 2014; 124(8): 1251-1258.
- Dale DC: How I diagnose and treat neutropenia. Current opinion in hematology 2016; 23(1): 1-4.
- Palmblad J, et al: How we diagnose and treat neutropenia in adults. Expert review of hematology 2016; 9(5): 479-487.
- Dale DC, Bolyard AA: An update on the diagnosis and treatment of chronic idiopathic neutropenia. Curr Opin Hematol 2017; 24(1): 46-53.
- Atallah-Yunes SA, Ready A, Newburger PE: Benign ethnic neutropenia. Blood reviews 2019; 37: 100586.
- Rappoport N, et al: The Duffy antigen receptor for chemokines, ACKR1,- ‘Jeanne DARC’ of benign neutropenia. British journal of haematology 2019; 184(4): 497-507.
- Reich D, et al: Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet 2009; 5(1): e1000360.
- Dale DC: Cyclic and chronic neutropenia: an update on diagnosis and treatment. Clinical advances in hematology & oncology 2011; 9(11): 868-869.
- Welte K, Zeidler C, Dale DC: Severe congenital neutropenia. Seminars in hematology. 2006; 43(3): 189-195.
- Andersohn F, Konzen C, Garbe E: Systematic review: agranulocytosis induced by nonchemotherapy drugs. Annals of internal medicine 2007; 146(9): 657-665.
- Garbe E: Non-chemotherapy drug-induced agranulocytosis. Expert opinion on drug safety 2007; 6(3): 323-335.
- Andres E, et al: Modern management of non-chemotherapy drug-induced agranulocytosis: a monocentric cohort study of 90 cases and review of the literature. European journal of internal medicine 2002; 13(5): 324-328.
- Kaufman DW, et al: Relative incidence of agranulocytosis and aplastic anemia. Am J Hematol 2006; 81(1): 65-67.
- Tesfa D, Keisu M, Palmblad J: Idiosyncratic drug-induced agranulocytosis: possible mechanisms and management. American journal of hematology 2009; 84(7): 428-434.
- Huguley CM: Agranulocytosis induced by dipyrone, a hazardous antipyretic and analgesic. JAMA 1964; 189: 938-941.
- Ibanez L, et al: Agranulocytosis associated with dipyrone (metamizole). European journal of clinical pharmacology 2005; 60(11): 821-829.
- Hedenmalm K, Spigset O: Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole). European journal of clinical pharmacology 2002; 58(4): 265-274.
- Shah RR: Metamizole (dipyrone)-induced agranulocytosis: Does the risk vary according to ethnicity? J Clin Pharm Ther 2019; 44(1): 129-133.
- Fauler J: Undesired effects of NSAIDS and coxibs. MMW Advances in Medicine 2005; 147(31-32): 31-35.
- Stamer UM, et al: Dipyrone (metamizole): Considerations on monitoring for early detection of agranulocytosis. Pain 2017; 31(1): 5-13.
- Njue L, Baerlocher GM: Drug-induced neutropenias/agranulocytoses. the informed physician 2018; 02/2018: 23-26.
- Beauchesne MF, Shalansky SJ: Nonchemotherapy drug-induced agranulocytosis: a review of 118 patients treated with colony-stimulating factors. Pharmacotherapy 1999; 19(3): 299-305.
- Sprikkelman A, de Wolf JT, Vellenga E: The application of hematopoietic growth factors in drug-induced agranulocytosis: a review of 70 cases. Leukemia 1994; 8(12): 2031-2036.
- Higgins P, et al: Rates of neutropenia in adults with influenza A or B: a retrospective analysis of hospitalized patients in South East Queensland during 2015. Intern Med J 2016; 46(11): 1328-1332.
- Dale DC, et al: A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood 1993; 81(10): 2496-2502.
- Starkebaum G: Chronic neutropenia associated with autoimmune disease. Seminars in hematology 2002; 39(2): 121-127.
- Morjaria S, et al: The Effect of Neutropenia and Filgrastim (G-CSF) in Cancer Patients With COVID-19 Infection. medRxiv 2020.
- Nawar T, et al: Granulocyte-colony stimulating factor in COVID-19: Is it stimulating more than just the bone marrow? Am J Hematol 2020; 95(8): E210-E3.
- Taha M, Sharma A, Soubani A: Clinical deterioration during neutropenia recovery after G-CSF therapy in patient with COVID-19. Respiratory medicine case reports 2020; 31: 101231.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(1): 5-9.