Pain management in patients with severe chronic kidney disease is no trivial matter due to limiting factors. Not only potential side effects can make the use of the painkillers also the potential for interaction with other drugs must be taken into account.
When thinking about kidney and painkillers, the famous analgesic nephropathy (also known as phenacetin kidney) may come to mind first. This chronic tubulointerstitial nephritis with papillary necrosis, which was first described in Switzerland [1], often led to terminal renal failure. However, due to the Europe-wide ban in the 1990s of combination painkillers containing phenacetin and addictive substances such as codeine, this disease has virtually disappeared and will therefore not be the subject of this article.
The interdependence of kidney (or renal function) and analgesics is complex (Fig. 1). On the one hand, it includes the direct nephrotoxic effect of certain classes of analgesics (e.g., non-steroidal anti-inflammatory drugs, NSAIDs), and on the other hand, the influence of a relevantly impaired renal function (e.g., many opioids) on the pharmacokinetics of certain analgesics. Last but not least, pain and renal insufficiency are closely related to multimorbidity/polypharmacy. In a study of over 400 general internist patients at a tertiary center, the combination of chronic renal failure, chronic pain (back and/or large joints), and arterial hypertension was the top multimorbidity cluster [2]. On the other hand, a recent American study showed that the patients with chronic renal failure suffered most from multimorbidity and polypharmacy [3].
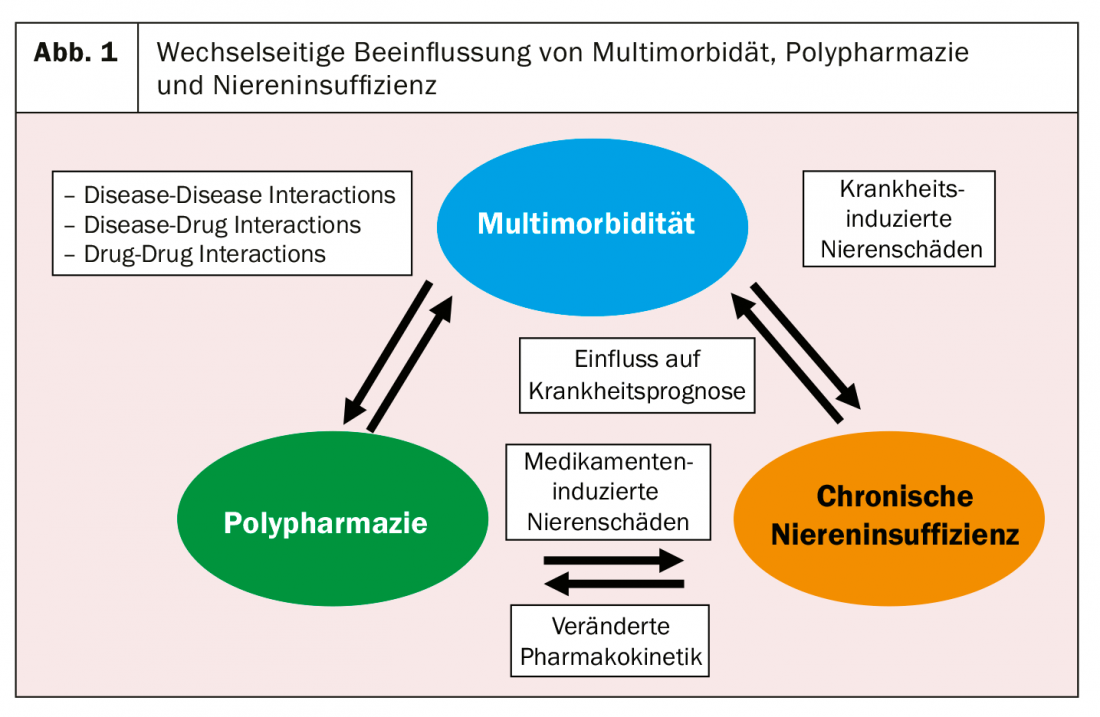
In this article, we would first like to provide an overview of the mutual influence of the kidney and analgesics, then go into more detail on the topics of nephrotoxicity and pharmacokinetics, and finally provide a practical recommendation for pain therapy in patients with chronic renal insufficiency.
Pain therapy according to WHO – Influence of the kidney
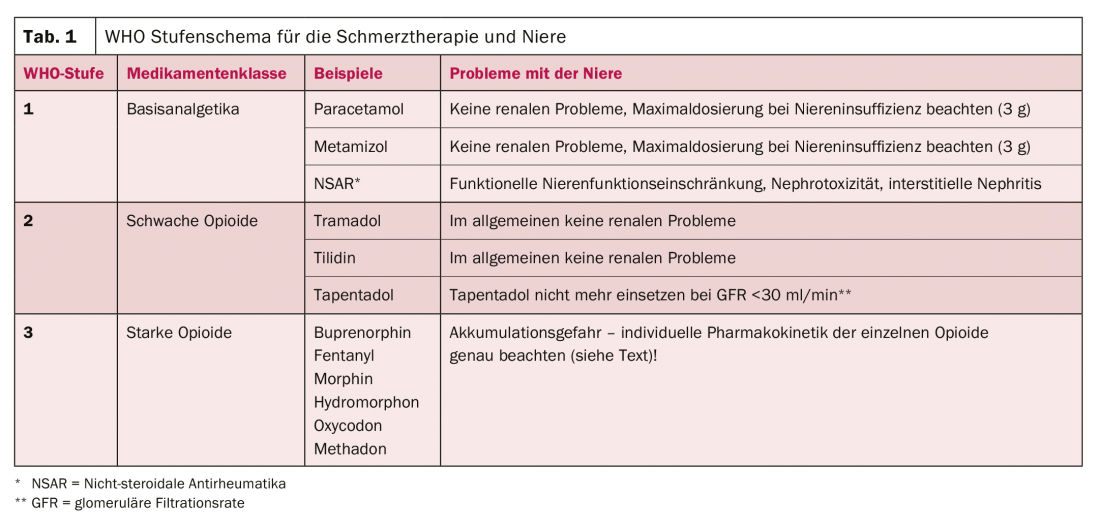
Pain therapy is usually planned according to the WHO stepwise scheme, which must always be adapted to the individual disease situation [4]. If we look at this scheme with the “kidney optics”, then the following statements can be made (Tab. 1, [5]):
- WHO Level 1: The basic analgesics paracetamol and metamizole offer few renal problems; in contrast, NSAIDs are potentially damaging to the kidneys in several ways, which will be discussed in more detail in the next section.
- WHO Level 2: The weak opioids also offer few renal problems, although tapentadol should not be used above a glomerular filtration rate (GFR) below 30 ml/min.
- WHO Level 3: Like the weak opioids, the strong opioids are not nephrotoxic. In contrast, there is a very different influence of renal function on their pharmacokinetics, which is why this must be closely observed in advanced renal insufficiency. The section after next is dedicated to this topic.
NSAIDs and kidney
NSAIDs affect the kidney in a variety of ways. They can lead to a functional limitation of the glomerular filtration rate, but also to structural damage. The functional decrease can be explained by the inhibition of prostaglandin synthesis (Fig. 2). Prostaglandins lead to vasodilation of the afferent arteriole in the glomerulum. Inhibition of these reduces glomerular perfusion. In contrast, blockers of the renin-angiotensin system (RAS) cause vasodilation in the efferent arteriole, which also leads to a decrease in perfusion pressure in the glomerulum. If dehydration with activated RAS is now clinically present and NSAIDs are then used in combination with RAS blockers, severe oliguric acute renal failure may result [6]. This is generally reversible but may progress to ischemic acute tubular necrosis if prolonged.
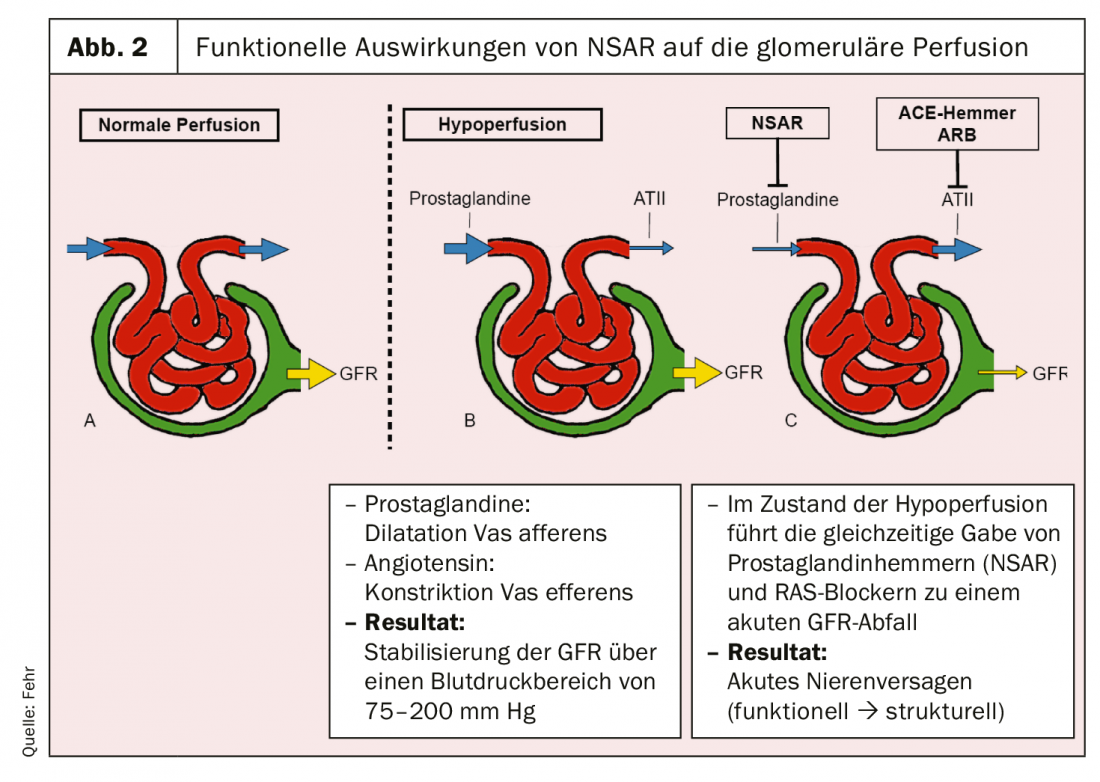
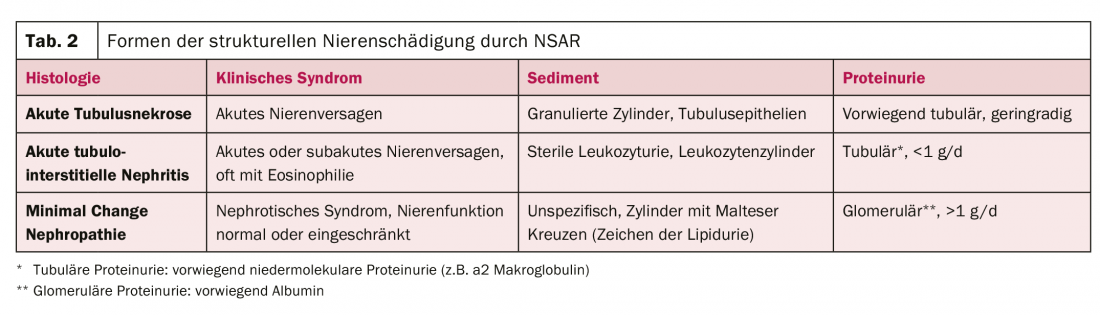
This brings us to the three structural patterns of damage that NSAIDs can cause to the kidney (Table 2):
- Acute tubular necrosis: Its development is primarily ischemic, as the final stage in the continuum of functional renal impairment described above. It has a typical course and recovers over a few days to weeks. So far, there is no specific therapy.
- Acute tubulointerstitial nephritis: acute tubulointerstitial nephritis is a drug-allergic reaction [7]. Biopsy typically shows an extensive tubulointerstitial infiltrate with edema formation. The renal tubules are destroyed in the process. Therapy consists primarily of discontinuing the causative agent. Corticosteroids are also used, but with only moderate evidence in the literature.
- Minimal change nephropathy: If severe proteinuria with signs of nephrotic syndrome (edema, effusions, hypoalbuminemia, hyperlipidemia) occurs after NSAID administration, minimal change nephropathy is the most likely diagnosis [8]. The diagnosis is made bioptically, and therapy consists of corticosteroids – in addition to discontinuation of the causative agent.
In summary, NSAIDs are excellent anti-inflammatory pain relievers. However, from a renal point of view, caution should be exercised in patients with pre-existing chronic renal insufficiency and in patients in a state of intravascular volume deficiency (dehydration, heart failure, liver cirrhosis).
Opioids and kidney
Opioids are generally not nephrotoxic. Their use in patients with chronic renal failure is limited primarily by pharmacokinetics. Many opioids or their active metabolites accumulate in advanced renal failure. The prototype for this is morphine, whose active metabolites morphine-3-glucuronide, morphine-6-glucuronide, and normorphine are excreted renally. For this reason, dose adjustment is necessary even in cases of mild renal impairment. Morphine should be discontinued in severe renal insufficiency (GFR <30 ml/min) [9].
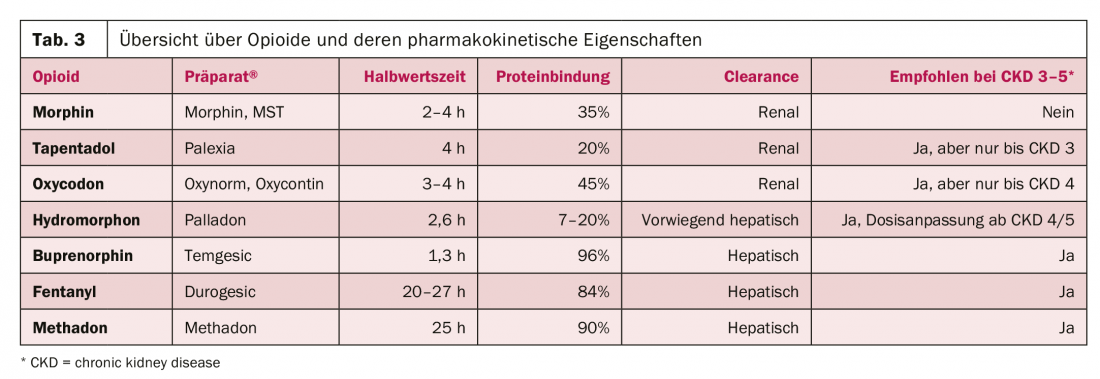
Table 3 provides an overview of the major opioids and their pharmacokinetic properties, as well as their recommendations for use in renal dysfunction. Three groups can be distinguished:
Opioids with predominantly renal elimination, which should be discontinued in patients with CKD 3-5 (morphine).
- Opioids with partial renal excretion, which should be used with caution and adjusted dose in patients with CKD 4/5 (hydromorphone, oxycodone, tapentadol).
- Opioids with high protein binding and hepatic elimination, which can be used relatively easily in patients with CKD 4/5 (buprenorphine, fentanyl, methadone).
Practice recommendations for the use of analgesics in patients with severe chronic renal failure (CKD 4/5, GFR <30 ml/min).
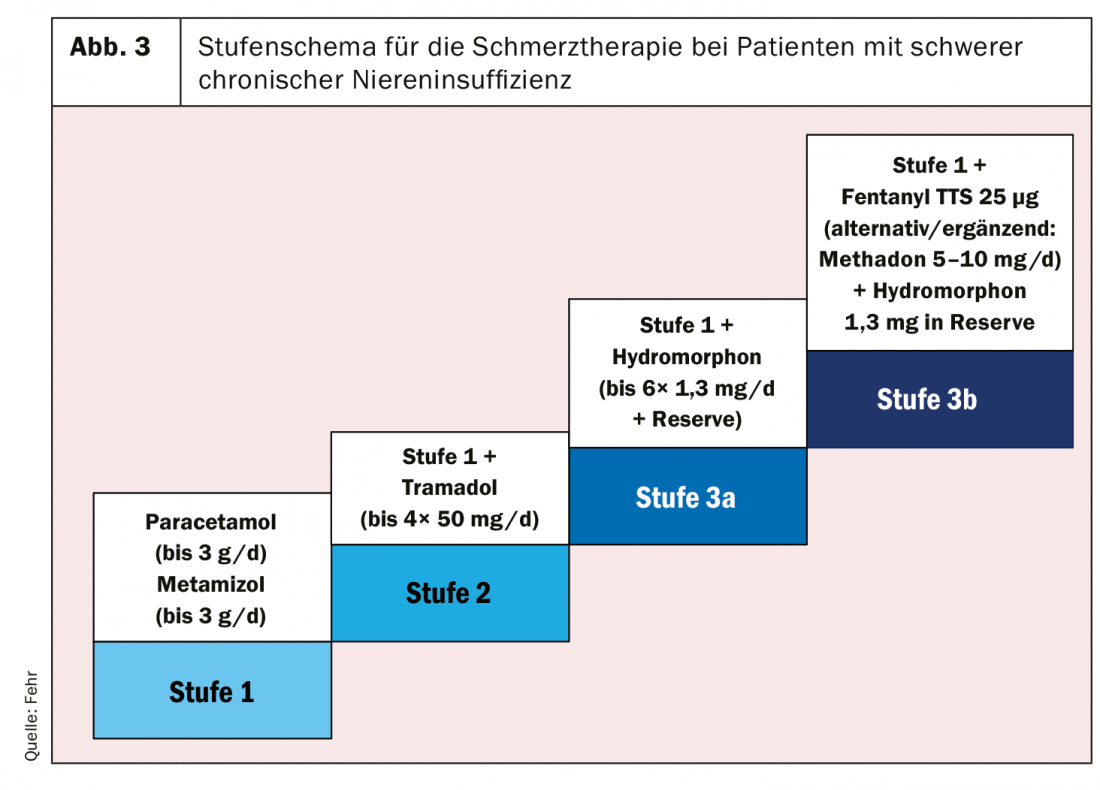
Based on the foregoing, we would like to propose here a stepwise scheme for the practice of pain management in patients with CKD 4/5 (Fig. 3) [5].
- In WHO stage 1, NSAIDs should be avoided whenever possible. The use of acetaminophen and metamizole is largely unproblematic, although the maximum dose should be limited to 3 (rather than 4) g/d.
- In WHO stage 2, we recommend using tramadol in the first instance. Its use in severe renal failure is largely unproblematic, but the remaining side effect profile (nausea, dizziness, hallucinations, etc.) often limits its use. Note the potential for interaction with drugs that inhibit the cytochromes CYP2D6 and CYP3A4.
- At WHO level 3, we recommend using primarily hydromorphone, which can be used by os and subcutaneously (if necessary, also in a subcutaneous pump). Fentanyl in patch application may be considered as an alternative. Both preparations can be combined with methadone (especially for neuropathic pain).
- When opioids are used, a WHO Level 1 drug should also generally be combined to achieve a synergistic effect in pain suppression. For neuropathic pain, we recommend using primarily gabapentin in addition to methadone (start with 50-100 mg/d, increase to a maximum of 300 mg/d) [10].
Literature:
- Zollinger HU. [Chronic interstitial nephritis caused by the abuse of anal getics containing phenacetin (Saridon etc.)]. Schweiz Med Wochenschr 1955; 85: 746.
- Siebenhuener K, Eschmann E, Kienast A, et al: Chronic Pain: How Challenging Are DDIs in the Analgesic Treatment of Inpatients with Multiple Chronic Conditions? PLoS One 2017; 12:e0168987.
- Tonelli M, Wiebe N, Manns BJ, et al: Comparison of the Complexity of Patients Seen by Different Medical Subspecialists in a Universal Health Care System. JAMA Netw Open 2018; 1:e184852.
- Caraceni A, Hanks G, Kaasa S, et al: Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13:e58-68.
- Davison SN: Clinical Pharmacology Considerations in Pain Management in Patients with Advanced Kidney Failure. Clin J Am Soc Nephrol 2019; 14: 917-931.
- Cippa PE, Fehr T: Medicines that go to the kidneys. Family Practice 2010: 3.
- Nast CC: Medication-Induced Interstitial Nephritis in the 21st Century. Adv Chronic Kidney Dis 2017; 24: 72-79.
- Fogo AB: Quiz page. Acute interstitial nephritis and minimal change disease lesion, caused by NSAID injury. Am J Kidney Dis 2003; 42:A41, e1.
- Dean M: Opioids in renal failure and dialysis patients. J Pain Symptom Manage 2004; 28: 497-504.
- Finnerup NB, Attal N, Haroutounian S, et al: Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162-173.
CARDIOVASC 2019; 18(6): 18-20