Despite great advances in the management of rheumatoid arthritis, many patients do not respond satisfactorily (or adequately) to available treatments [1]. Data from clinical trials of a targeted disease-modifying antirheumatic drug show that with additional effective therapeutic options, more and more RA patients can also achieve the goal of remission in the long term [2, 3].
Rheumatoid arthritis (RA) is one of the most common chronic inflammatory diseases – affecting approximately 85,000 people in Switzerland alone [1, 4]. RA is typically characterized by progressive inflammation of the affected joints, which can lead to cartilage loss, irreversible bone erosion, and disability [1]. In addition, extra-articular manifestations occur in approximately half of patients, contributing to the increased mortality in RA [1, 5].
Timely and targeted treatment of RA [1].
Timely and targeted treatment is essential to counteract the considerable consequences of the disease and to enable patients to achieve remission or at least low disease activity [1]. If the therapeutic goal is not achieved by the use of a conventional synthetic disease-modifying antirheumatic drug (csDMARDs), such as methotrexate (MTX), and poor prognostic factors are present, treatment should be extended to include a drug from the group of biologic or targeted DMARDs, according to the EULAR 2020 recommendations [6]. The latter also include Janus kinase (JAK) inhibitors, which specifically interfere with the pro-inflammatory JAK signal transducer of activation (STAT) signaling cascade to suppress RA-typical inflammatory processes [1].
JAK inhibitor upadacitinib as a long-term effective therapeutic option [7].
The reversible, selective JAK inhibitor upadacitinib (RINVOQ®) was approved in January 2020 for the treatment of adults with moderate-to-severe active RA who have had an inadequate response to or have been intolerant of treatment with one or more csDMARDs [7, 8]. Upadacitinib can be taken as monotherapy or in combination with MTX as well as other csDMARDs once daily in tablet form (15 mg), with or without food [7]. Treatment costs are covered by health insurance [9]. The decisive factor for the approval of upadacitinib was the results of the RA-SELECT study program with a total of more than 4,380 adult patients with active RA in five pivotal studies [7]. Upadacitinib was shown to be an effective treatment option both in combination with MTX and as monotherapy [2, 3].
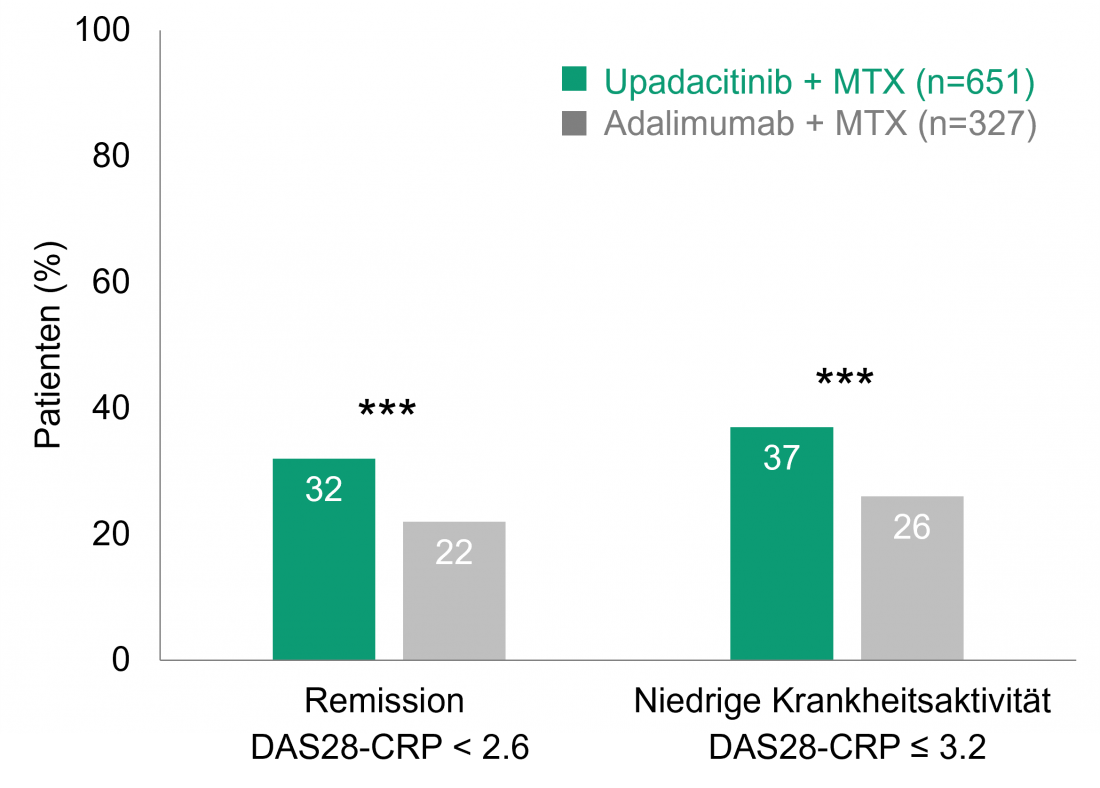
Upadacitinib + MTX versus adalimumab + MTX: Higher remission rates even after 156 weeks [2].
The placebo-controlled randomized phase III SELECT-COMPARE trial compared the efficacy and safety of upadacitinib and the TNF-alpha inhibitor adalimumab, both in combination with MTX. For this purpose, 1,629 patients who had responded inadequately to MTX received 15 mg upadacitinib (once daily), 40 mg adalimumab (biweekly), or placebo, all of them + MTX [10]. If patients responded inadequately to their assigned therapy, they were switched from placebo to upadacitinib, from upadacitinib to adalimumab, or from adalimumab to upadacitinib after 14 to 26 weeks; all remaining patients on placebo received upadacitinib starting at week 26 [11]. The initial 48-week, double-blind phase was followed by an open-label extension with 1,403 patients [2]. Already after 12 and after 48 weeks, significantly more patients achieved remission (Disease Activity Score for 28-joints C-Reactive Protein, DAS28-CRP < 2.6) or low disease activity (DAS28-CRP ≤ 3.2) under upadacitinib + MTX than under adalimumab + MTX [10, 11]. This superior efficacy of upadacitinib + MTX versus adalimumab + MTX persisted throughout the open-label extension observation period(Figure 1): After 156 weeks, 32% of patients with upadacitinib + MTX were in remission, compared with 22% in the adalimumab + MTX group (p < 0.001). Low disease activity was seen in 37% with upadacitinib + MTX and 26% with adalimumab + MTX (p < 0.001). Significantly greater improvements in the patient-related parameters of pain and physical functioning (Health Assessment Questionnaire-Disability Index, HAQ-DI) were also observed in the upadacitinib + MTX arm than in the comparison group after 156 weeks (p < 0.001 for pain and p < 0.01 for HAQ-DI) [2].
The challenge of polypharmacy [12]Polypharmacy, defined as the simultaneous use of five or more medications, is an increasing phenomenon – even among middle-aged people – associated with multimorbidity. Thus, it is associated with negative consequences such as falls, hospitalization, and even death. The inflationary use of non-essential drugs should thus be avoided. However, figuring out which additional medications provide more benefit than harm to patients is a major challenge [12]. |
Upadacitinib also effective as monotherapy [3].
The question of whether better results can be achieved with upadacitinib than with MTX alone, even without the additional use of csDMARDs, was the focus of the double-blind randomized phase III SELECT-MONOTHERAPY trial. For this purpose, the efficacy and safety of upadacitinib as monotherapy after switching from MTX compared with continued MTX treatment were evaluated in 648 patients with inadequate response to initial MTX treatment. At 14 weeks, significantly more patients on upadacitinib monotherapy achieved the primary endpoint, a 20% response according to American College of Rheumatology (ACR) criteria, than on MTX (68% vs. 41%, p ≤ 0.0001). In addition, a significantly greater proportion of patients from the upadacitinib group were in remission or showed low disease activity than with MTX (DAS28-CRP < 2.6: 28% vs. 8%; DAS28-CRP ≤ 3.2: 45% vs. 19%; both p ≤ 0.0001) [3].
Long-term safety profile of upadacitinib [13]
The European Medicines Agency (EMA) has currently initiated an evaluation of safety in the treatment of chronic inflammatory diseases for all approved JAKi [14]. The safety profile of upadacitinib in long-term RA compared with MTX and adalimumab was assessed in an integrated analysis of the five pivotal phase III studies in the SELECT trial program. This included 2,630 patients on at least one dose of 15 mg upadacitinib; more than half of the patients took upadacitinib for at least 48 weeks. In total, this corresponds to an analysis of 4,020.1 patient-years. Adverse events leading to treatment discontinuation occurred with comparable frequency among upadacitinib, MTX, and adalimumab (upadacitinib 8.4; MTX 9.5; adalimumab 11.1 per 100 patient-years). The incidence of serious infections was similar in the upadacitinib and adalimumab groups but higher than in the MTX group. Opportunistic infections, venous thromboembolism, major adverse cardiac complications (MACE), and malignancies occurred with comparable frequency in the three groups. Herpes zoster was more common with upadacitinib than with adalimumab and MTX (upadactinib: 3.7; MTX: 1.4; adalimumab: 1.3 per 100 patient-years), but was not severe in 96% of cases. Overall, no new safety signals were observed with upadacitinib in the long-term integrated analysis [13].
Conclusion
Upadacitinib (RINVOQ®) offers an effective therapeutic option with a favorable benefit-risk profile in moderate to severe active RA and inadequate response or intolerance to at least one csDMARD [7, 16]. As shown by the long-term analysis of the SELECT COMPARE study, daily use of the reversible and selective JAK inhibitor over a period of more than 3 years can enable more than 30% of treated patients to achieve the therapeutic goal of remission (DAS28-CRP < 2.6) [2]. Moreover, most RA patients prefer treatment in tablet form and without the additional use of MTX, thus the option of oral (mono)therapy with upadacitinib is potentially associated with improved adherence and may counteract polypharmacy [12, 16].
Literature
The references can be requested by professionals at medinfo.ch@abbvie.com.
Brief technical information RINVOQ®
This text was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, 6330 Cham.
CH-RNQR-220045_04/2022
Contribution online since 15.04.2021
Post updated 30.05.2022