The treatment of rheumatoid arthritis (RA) has made great progress over the past 20 years. This relates both to the range of drugs available and to developments in terms of rapid onset of action and response rates. Phase III clinical trials were published in 2019, particularly for JAK inhibitors, and the results were presented at the Rheumatism Update in Wiesbaden.
The 2019 update recommendations from the European League Against Rheumatism (EULAR) show few differences from previous editions. Relevant is the recommendation to use at most a second TNF blocker in case of therapy failure on the first TNF blocker. JAK inhibitors continue to be positioned after treatment failure on csDMARDs or biologics. This can be formally interpreted to mean that switching from one JAK inhibitor to another is possible and reasonable in the event of treatment failure, explained Prof. Andrea Rubbert-Roth, M.D., Department of Rheumatology, Cantonal Hospital St. Gallen. However, no data is yet available for this. In view of the recent approval of upadacitinib and soon probably also filgotinib, the question of an optimal switching strategy arises, especially for the JAK inhibitors.
Four JAK inhibitors available for RA
Last year, peficitinib received approval for the Japanese market. Thus, there are currently four approved JAK inhibitors for the treatment of RA, although peficitinib has not yet been approved for use in the United States or Europe. The expert focused on the question of substance-specific differences in efficacy and safety profile. In view of the difficult handling (necessity of cooling, parenteral administration), it must be stated that biologics do not represent the optimal form of therapy for all patients, especially since only a minority achieve continuous clinical remission. Pain, functional limitations, fatigue and depression, on the other hand, represent a high disease burden for those affected.
The selectivity of JAK inhibitors, i.e. the inhibition of individual isoforms at a certain intracellular concentration, is the main focus of investigations. The optimal ratio of effectiveness and side effects, such as anemia, is thus sought. The phase III clinical trial program for tofacitinib and baricitinib has been completed; for upadacinitib, the results of a head-to-head study with abatacept in RA patients who have not responded adequately to previous biologic therapy remain to be seen.
Tofacitinib
There is not much new in terms of efficacy with tofacitinib, as it has been available and well known in the US for many years. Tofacitinib has also been approved in Switzerland since 2013. A new post-hoc analysis regarding seropositive and seronegative patients has shown a comparable response in both groups. DAS28 remission rates were slightly lower than in CCP patients.
In the analysis, data from five phase III studies were pooled and subgroups were defined based on serology: RF+CCP+, RF+CCP-, RF-CCP+, and RF-CCP-. As a result, patients with RF+CCP+ had a higher likelihood of achieving an ACR-20, -50, or -70 response than RF-CCP patients. This result was demonstrated for ACR-20 and -50 response for both tofacitinib 5 mg twice daily and tofacitinib 10 mg twice daily. In contrast, the ACR-70 response was better only at the higher dose [1].
Another study looked at the risk of cardiovascular events with tofacitinib. Analysis of a recent phase IV study comparing tofacitinib with TNF blockers (adalimumab, etanercept) in patients older than 50 years with at least one additional risk factor for cardiovascular events showed that the risk for thromboembolic events associated with tofacitinib should be considered increased.
The risk of pulmonary embolism was significantly increased with tofacitinib at the 10-mg dose and was also higher with 5 mg twice daily than in previous studies. Also, all-cause mortality was higher under 10 mg twice daily than under 5 mg twice daily. The EMA subsequently issued a recommendation that tofacitinib should not be used in patients at increased risk of thromboembolism. RA patients over 65 years of age should be treated with tofacitinib only if there is no therapeutic alternative. Prof. Rubbert-Roth commented that she finds the EMA recommendation based on a study that has not yet been scientifically presented and discussed “unusual” and hopes that this will now happen soon.
Baricitinib
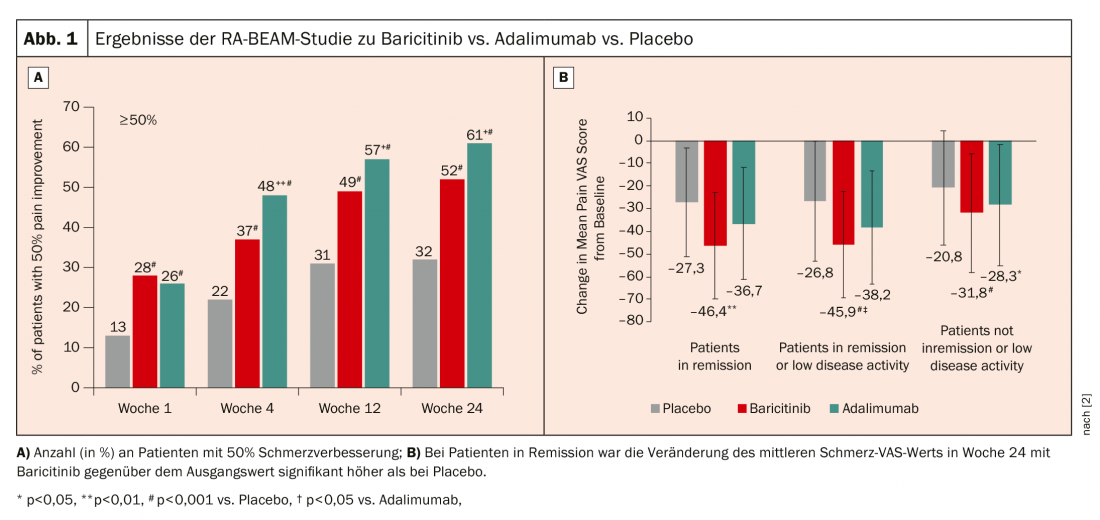
Baricitinib has been approved in Switzerland since 2017 at a dose of 4 mg (2 mg in elderly patients or in renal insufficiency). In the RA-BEAM trial, baricitinib 4 mg + MTX was compared with adalimumab + MTX and placebo + MTX [2]. Here, the 50% improvement in pain has been found to be better with baricitinib (Fig.1A). Nevertheless, when looking more closely at only those patients who are in remission or low disease activity, baricitinib has a significantly meaningful effect on pain (Fig.1B). The rheumatologist can only speculate as to why this is so: “It is possible that pain receptors also use JAKs in their signaling, which means that they then also have specific pain effects.” The positive effect could also be observed after a switch from adalimumab to baricitinib after a week. 16 determine.
Regarding vaccination response under baricitinib, PCV13 and tetanus toxoid (TT) have been tested. 106 Patients from the RA-BEYOND trial of 2 mg or 4 mg baricitinib (with and without MTX) were vaccinated with PCV13 and against tetanus. Overall, 68% of patients showed adequate vaccine response to pneumococcal disease, and 43% achieved an increase in vaccine titers of more than 4-fold to tetanus. An increase of more than 2-fold after PCV13 was achieved by 74% of patients [3].
Upadacitinib
According to Prof. Rubbert-Roth, one of the most important studies in the past year is SELECT-COMPARE, which compared upadacitinib vs. adalimumab vs. placebo in MTX-IR patients with active RA [4]. In this study, there was an option to switch from upadacitinib to adalimumab at week 12 as well as vice versa (at weeks 14, 18, 22, and 26) if there was less than 20% improvement in painful and swollen joints (co-primary endpoints ACR20 and DAS28-CRP <2.6).
In the study of 1629 patients with active RA despite MTX, upadacitinib (approved in Switzerland since spring 2020) was shown to be statistically significantly more effective than adalimumab: 71% of patients achieved an ACR20 response with upadacitinib, compared with only 63% with adalimumab and 36% with placebo. 29% of patients achieved remission according to DAS28-CRP, compared with only 18% on adalimumab and 6% on placebo. In terms of adverse events, herper zoster and CK rises were more common with upadacitinib; venous thromboembolism (VTE) occurred in 3 patients with adalimumab vs. 2 patients with upadacitinib. Looking at the non-responders, it was seen that when switching from upadacitinib to adalimumab, 13% achieved remission, while conversely, switching from adalimumab to upadacitinib achieved the desired outcome in 26%.
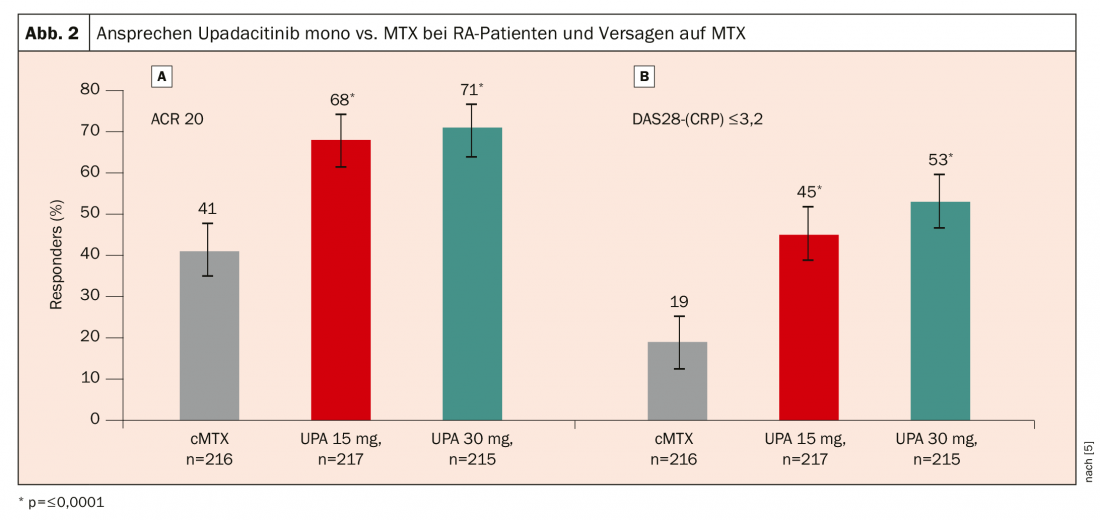
In the SELECT-MONOTHERAPY trial, 648 patients with active RA despite MTX were randomized to either continuation of MTX therapy or upadacitinib 15 mg or 30 mg in monotherapy [5]. Again, a significantly better response was seen with upadacitinib: after 14 weeks, an ACR20 response was achieved in 68% of patients on upadacitinib 15 mg and 71% on upadacitinib 30 mg, compared with only 41% of patients who continued MTX therapy (Fig. 2).
Filgotinib and peficitinib
Filgotinib is considered a selective JAK1 inhibitor, and the phase III FINCH1, -2, and -3 trial program has been published or presented at international meetings in 2019. FINCH2 evaluated the efficacy and safety of filgotinib (100 mg or 200 mg daily) compared with placebo for 24 weeks in 449 patients with active RA despite stable therapy with csDMARDs and prior treatment failure or intolerance among biologics (bDMARDs) [6]. 23.4% of patients had three or more prior bDMARDs; the primary endpoint was achievement of ACR20 response at 12 weeks. The result here was that 66% met the primary endpoint on filgotinib 200 mg, 57.5% on filgotinib 100 mg, and 31.1% of placebo patients. In patients with treatment failure to at least three bDMARDs, ACR20 response was 70.3% on 200 mg filgotinib, 58.8% on 100 mg, and 17.6% on placebo. “This will certainly also be a substance that will soon go into regulatory approval,” Prof. Rubbert-Roth assessed the figures.
The expert, on the other hand, does not seem as convinced by peficitinib, which will be approved in Japan in 2019. She referred only to a study that looked at pan-JAK inhibitor versus placebo for 52 weeks in patients with active RA despite conventional DMARDs but without recording radiographic progression. 507 patients were randomized. Peficitinib showed an ACR20 response of 57.7% at 12 weeks with peficitinib 100 mg and 74.5% with peficitinib 150 mg vs. 30.7% with placebo (p<0.001). However, an open-label comparator arm with etanercept showed that etanercept consistently outperformed peficitinib, achieving an ACR20 response of 83.5%.
Source: Rheumatism Update, Wiesbaden (D)
Literature:
- Bird P, Hall S, Nask P, et al: RMD open 2019; 5: e000742; doi:10.1136/rmdopen-2018-000742. (Epub ahead of print).
- Fautrel B, Kirkham B, Pope JE, et al: J Clin Med 2019; 8: 1394-408.
- Winthrop K, Bingham CO, Komocsar WJ, et al: Arthritis Res Ther 2019; 21: 102-112.
- Fleischmann R, Pangan AL, Song ICH, et al: Arthritis Rheumatol 2019; 71 (11): 1788-1800.
- Smolen JS, Pangan AL, Emery P, et al: Lancet 2019; 393: 2303-2311.
- Genovese MC, Kalunian K, Gottenberg JE, et al: JAMA 2019; 322 (4): 315-325.
InFo PAIN & GERIATRY 2020; 2(1): 32-35 (published 5/7/20, ahead of print).