In the treatment of non-small cell lung cancer, an extremely large amount has been achieved in a short time. Keeping track of so many rapid innovations can be quite difficult, but it’s worth it. This is because patients can already benefit enormously from the new treatment approaches.
Classically, there were clear guidelines for the therapy of non-small cell lung cancer depending on the TNM stage. These are currently being increasingly challenged by the development of new, targeted therapies such as 3rd generation tyrosine kinase inhibitors or immunotherapeutics. An example of this is the new recommendation to perform maintenance therapy with a checkpoint inhibitor for one year after radiochemotherapy in cases of stage III PD-L1 expression [1].
News in neoadjuvant therapy
Not only are there new treatment approaches for maintenance therapy in stage III, but exciting new options are also in the pipeline for neoadjuvant therapy. For example, it was recently discovered that after only two neoadjuvant administrations of a PD-L1 inhibitor, many patients show a significant proportion of dead tumor cells in the resectate, and this is despite the fact that a response is not necessarily detectable radiologically [2]. Since the risk of recurrence increases significantly with the proportion of living tumor cells in the removed tissue [3], this finding is of great relevance for therapy and shows that checkpoint blockade could at least represent a valid alternative to chemotherapy in neoadjuvant therapy as well. Several drugs are currently being tested for this application with promising results so far. Even in stage IIIA, positive effects of perioperative immunotherapy in addition to neoadjuvant chemotherapy have been shown [4].
Molecular diagnostics
Considering the potential benefit of tyrosine kinase inhibitor or immunotherapy even in early tumor stages, the current guidelines for molecular diagnostics seem rather cautious. Analysis of PD-L1 expression is recommended in stages III and IV, testing of common mutations for which approved targeted therapies exist only in stage IV (Tab. 1). This concept certainly needs to be questioned in the current data situation, where, for example, the use of tyrosine kinase inhibitors such as osimertinib as adjuvant therapy in EGFR mutated tumors of lower stages raises justified hopes [5, 6].
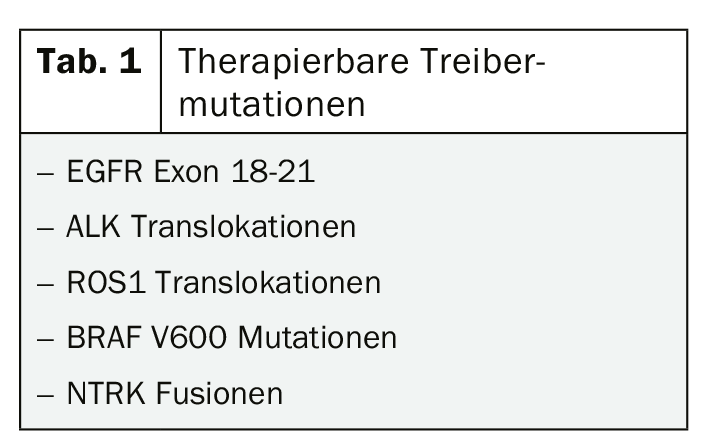
Although the new options for the treatment of mutant NSCLC in particular have led to some euphoria and molecular testing is likely to be expanded in the near future, it is important to keep in mind that 80% of NSCLC do not have a treatable driver mutation. However, in those patients in whom a targetable mutation can be detected, targeted therapy is the best choice, at least in stage IV. Accordingly, much has happened in this area in recent months with new approvals of the ALK inhibitors brigatinib and lorlatinib, among others.
What to do if there is no driver mutation?
In the more common case where no driver mutation can be detected in stage IV NSCLC, there are unfortunately fewer therapeutic options. However, immunotherapeutics are also increasingly used for these tumors. When PD-L1 expression exceeds 50%, primary use has been checkpoint inhibitors, which are clearly superior to chemotherapy in this patient group. Moreover, regardless of PD-L1 expression, the combination of immunotherapy and chemotherapy was shown to be more effective than chemotherapy alone [7].
Recently, additional concepts are also emerging for the treatment of advanced NSCLC without a treatable driver mutation, such as the combination of two immunotherapies independent of PD-L1 expression level [8]. This approach appears clearly superior to chemotherapy alone, but has not yet been compared with the combination of immunotherapy and chemotherapy. Based on the data to date, PD-L1 expression is not a mandatory requirement for response to immunotherapy, particularly intensified immunotherapy with different drug classes.
Apart from other therapeutic combinations, new targets have recently become known. These include TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domain), which is frequently expressed together with PD-L1. A study combining an appropriate inhibitor with PD-L1 blockade provided first promising results [9].
The right lid for the right pot
Proper and most efficient patient selection for the multitude of available therapies remains a sticking point. Increasingly specific therapies and smaller target groups contribute to the importance of predictive markers and reliable molecular genetic diagnostics. Even though the pipeline is bubbling, longer-term data are still lacking for many of the new substances and combinations. Thus, new questions, hurdles, but also many more possibilities emerge at all corners and ends. In short: There is only one thing that helps – stay tuned!
Source: Forum for Continuing Medical Education (FOMF), Refresher, Immunoncologics and Targeted Therapies – Presentation on “Non-Small Cell Lung Cancer,” Livestream June 20, 2020, PD Dr. Niels Reinmuth, Chief of Oncology at Asklepios Clinic.
in Munich-Gauting (D).
Literature:
- Antonia SJ, Villegas A, Daniel D, et al: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. New England Journal of Medicine. 2017;377(20): 1919-1929.
- Forde PM, Chaft JE, Smith KN, et al: Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21): 1976-1986.
- Hellmann MD, Chaft JE, William WN, Jr, et al: Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1): e42-50.
- Rothschild S, Zippelius A, Savic S, et al: SAKK 16/14: Anti-PD-L1 antibody durvalumab (MEDI4736) in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC)-A multicenter single-arm phase II trial. Journal of Clinical Oncology. 2016;34(15_suppl): TPS8573-TPS.
- Zhong WZ, Wang Q, Mao WM, et al: Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1): 139-148.
- Wu YL, Herbst RS, Mann H, et al: ADAURA: Phase III, Double-blind, Randomized Study of Osimertinib Versus Placebo in EGFR Mutation-positive Early-stage NSCLC After Complete Surgical Resection. Clin Lung Cancer. 2018;19(4): e533-e536.
- Jotte RM, editor IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC2018; ASCO Annual Meeting: American Society of Clinical Oncology.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al: Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. New England Journal of Medicine. 2019;381(21): 2020-2031.
- Rodriguez-Abreu D, Johnson ML, Hussein MA, et al: Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). Journal of Clinical Oncology. 2020;38(15_suppl): 9503.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(4): 30-31 (published 9/20/20, ahead of print).
InFo PNEUMOLOGY & ALLERGOLOGY 2020, 2(3): 34-35.