Patients with refractory cardiovascular failure are dependent on mechanical circulatory support. When do short-term systems such as ECMO or Impella come into play? In which cases is long-term support provided?
Modern mechanical cardiovascular support enables us to offer therapy concepts to patients with completely refractory cardiovascular failure that would have been unthinkable ten years ago. Technical developments over the past 30 years have led to a clear focus on individual forms of therapy in everyday life, enabling the treating physician to offer patients proven systems with a clear conscience. What was recently an almost experimental field of sometimes adventurous-looking equipment is becoming more and more manageable. Thus, in this article, we will skip the review of outdated aggregates and provide you with a concise summary of current therapeutic concepts in the complete failure of conventional heart failure therapy.
What are mechanical circuit support systems?
This is a generic term for all systems that support or replace the blood circulation. A distinction is made between short- and long-term systems. Short-term systems are devices that are external to the body and have a limited duration of use. If the heart does not recover, long-term systems are used, which today generally consist of a small pump that the cardiac surgeon implants directly in the patient’s heart. This pump is connected by a cable to a control unit and batteries outside the body. Thus, the frequently used term “artificial heart” does not fully reflect the technical reality.
Patient group in focus
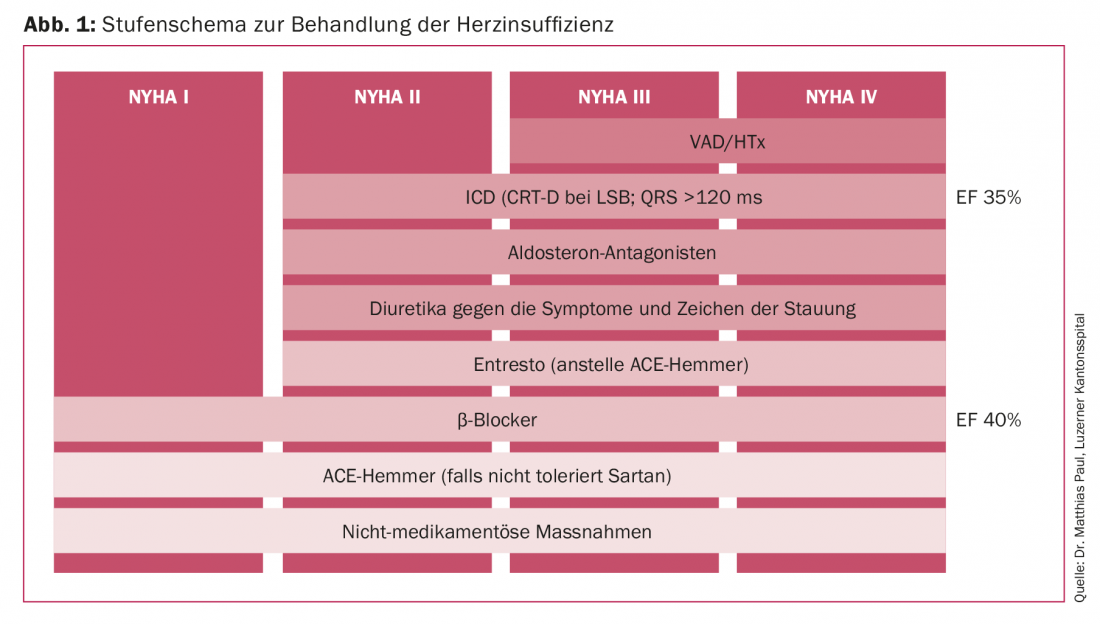
If we look at the staged scheme for the treatment of heart failure listed here (Fig. 1), the patient population being treated is on the top tier. After steadily increasing numbers, especially in the area of long-term support systems, new drug strategies with sacubitril/valsartan and levosimendan have in the meantime led to patients being able to be stabilized conservatively for longer and to a decrease in implantation numbers. New sophisticated resynchronization therapies have a similar effect. To what extent this is just a current trend due to the deferring effect of the new forms of therapy remains to be seen.
To further break down the patient population to be discussed here, INTERMACS can be used in the area of mechanical circulatory support. The Interagency Registry for Mechanically Assisted Circulatory Support is an annually published North American database that documents all outcomes of support systems implanted in North America [1]. INTERMACS profiles derived from these data allow the treating physician to further classify terminally refractory heart failure and match the optimal use for mechanical cardiac support (Fig. 2).
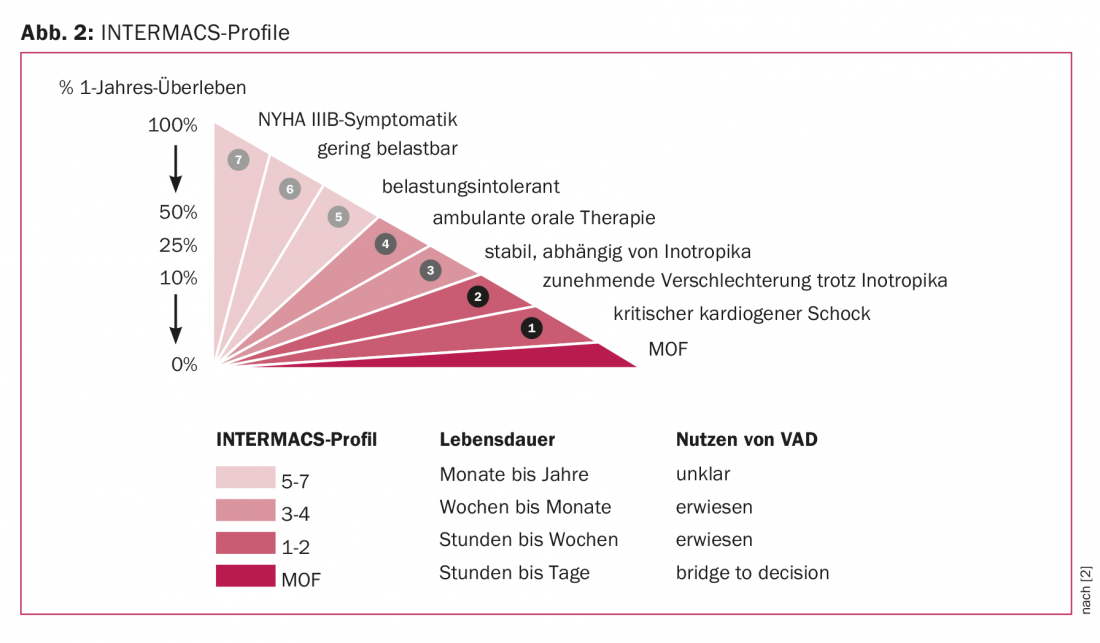
According to this classification, terminally heart failure patients are divided into eight profiles [2]. While INTERMACS profile 8 describes the ambulatory patient with advanced dyspnea, profile 1 corresponds to the dying patient. Intermediate gradations distinguish ambulatory patients from increasingly catecholamine-requiring patients. Ideal implantation times can be defined using the specifications of these profiles. Consequently, one can also speak of a too early or simply missed point in time. In the same way, the degree of disease dictates our choice of device. While patients in a stable condition can be supported with long-term systems, the implantation of which should be discussed with the patient and relatives beforehand, patients in a completely unstable condition are provided with short-term support systems, the primary aim of which is to keep the patient alive in the first place.
Short-term opportunities
Veno-arterial extracorporeal membrane oxygen ation (“Veno Arterial Extracorporeal Membrane Oxygenation”, VA ECMO) can be used in the short term. This enables rapid circulatory support or complete circulatory replacement in situations where acute heart failure occurs. VA ECMO ensures an adequate supply of oxygen to the organs in cardiogenic shock and, at the same time, the removal of accumulated metabolic waste products. Due to the veno-arterial insert with oxygenator, oxygenation is also ensured, which also provides acute relief to the lungs and right circulation. The advantage of ECMO is the relatively “simple” implantation and placement via percutaneous peripheral access, ideally ultrasound-guided punctured or – if available – under fluoroscopy. This can also be done under continuous resuscitation. In addition to cardiac support, another advantage is the ability to oxygenate the blood, since fulminant cardiovascular failure often involved the lungs. As a disadvantage of the system, the lack of relief for the heart must be mentioned. Even if function of the heart and lungs can be effectively taken over, the heart muscle does not recover effectively under VA ECMO therapy because of the increase in afterload.
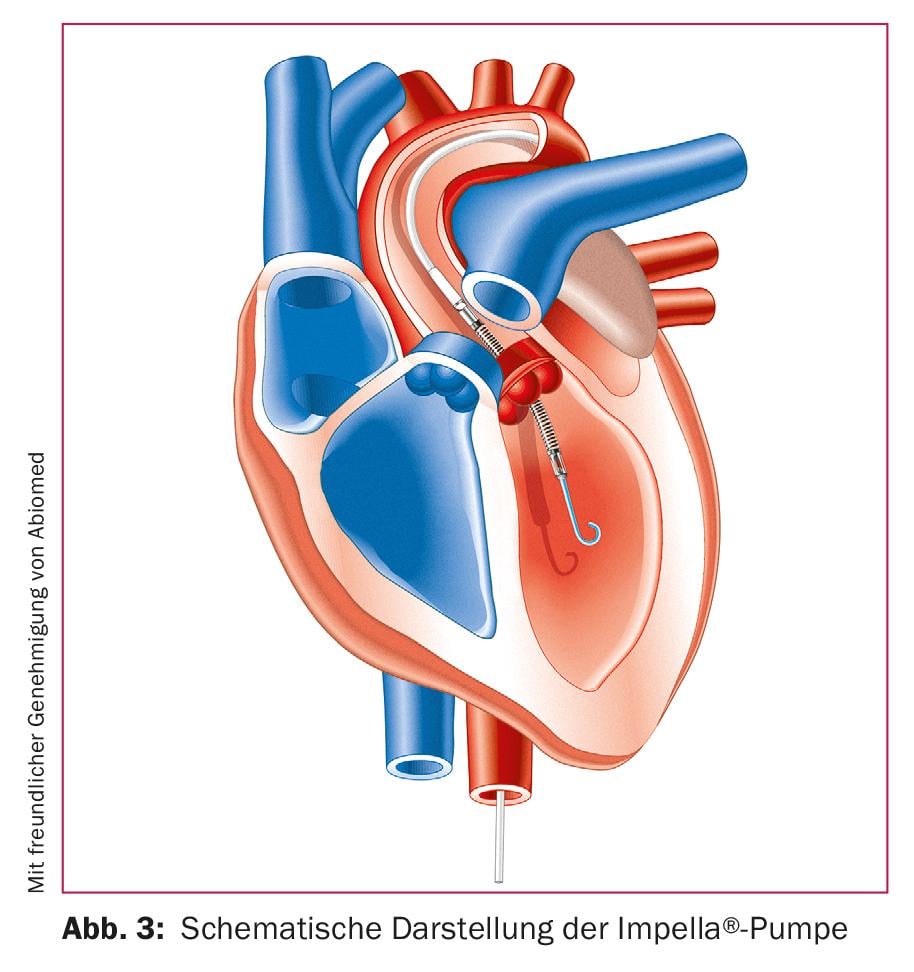
The Impella® pump systems are microaxial pumps suitable for left and right heart support (Fig. 3). The systems available for the left heart are generally placed retrograde from the femoral artery via the aortic valve in the left ventricle. At up to 50,000 revolutions per minute, the pump draws blood into the left ventricle and ejects it into the ascending aorta. This happens continuously and independently of the pulse rate. The microaxial pump available for the right heart works on the same principle. Inserted via the femoral vein, it connects the inferior vena cava to the pulmonary artery via the tricuspid and pulmonary valves, delivering blood to the pulmonary artery. The clear advantage of Impella® systems is the total and continuous unloading of the heart, which is considered a prerequisite for adequate cardiac recovery. The disadvantages are the more complex placement under fluoroscopy and the lack of oxygenation. For this reason, modern therapy concepts combine VA ECMO and Impella® systems in the same patient.
Long term support
Two systems are currently considered the benchmark for implantation of long-term support systems: the HeartWare HVAD® from Medtronic and the HeartMate 3® from Abbott [3,4] (Fig 4). Both systems are fully magnetic bearing centrifugal flow pumps. If the heart is not pumping properly or only to a limited extent, the implantation of such a ventricular assist device (VAD) can maintain the supply of sufficient blood to the body. In most cases, the system is used to support the left ventricle. The pump can be surgically connected to the ventricle via an opening of the chest due to its small size within the pericardium. At the apex of the heart, the aggregate draws in oxygen-rich blood and releases it into the aorta. Despite increasing miniaturization, the systems are capable of pumping eight to ten liters. A cable is connected to the pump and connects the system to a control unit and batteries, which in the basic battery configuration provide the patient with up to eight hours of independence from power sources.

Support systems in everyday hospital life
In everyday clinical practice, patients with acute, non-stabilizable heart failure are initially treated with short-term systems. In addition to stabilizing the patient, this also saves time, enabling the treating team to analyze the patient’s social environment, to find out whether life-prolonging measures were desired by the patient at all, and to assess whether cerebral functions compatible with further life can be expected at all after resuscitation. Ideally, stabilization should be achieved using ECMO and Impella® systems, allowing extubation of the patient and face-to-face discussion.
Implantation of a long-term system in the acute setting is not practical because of the high mortality in this situation, the relative irreversibility of the procedure, and the associated costs, and because of the availability of alternative short-term systems today.
Ultimately, clinical practice has shown us that only a fraction of patients at the short-term support system are ever provided with a long-term system. Most patients die as part of the overall situation, with a small proportion experiencing cardiac recovery that does not necessitate at least peracute care with a long-term system. An even smaller percentage will receive the “upgrade” to a definitive system.
The INTERMACS profiles allow calculation of the ideal time for implantation of a long-term support system. Completely unstable patients should receive long-term systems only through short-term support, if at all. In principle, the timing for implantation of long-term devices can be either too late or too early. Despite a noticeable reduction of everyday restrictions, e.g. shortness of breath, immobility and dependence on the hospital, the implantation of an “artificial heart” also entails a certain morbidity, which, however, must not exceed that of the underlying disease.
While mortality during surgery is now very low, the interaction of the flow pump with the patient’s blood and the transfer of data and energy via a cable passing through the skin potentially poses some dangers. In addition to emboli from inadequate anticoagulation, overly harsh settings and consumption of clotting factors by contact with nonphysiologic surfaces lead to bleeding in the gastrointestinal system and brain. The penetration point at the skin surface as well as the system per se provide a target for infections. The annual risk of major infections and bleeding or embolism is between 8 and 10%. Mortality after implantation over 30 days is 3% [4].
What is the role of heart transplantation?
Heart transplantation must still be considered the gold standard for the treatment of refractory heart failure. Nowadays, cardiac assist devices are supplied in the acute state with short-term systems. Long-term systems are used for poorly treatable heart failure when patients on the increasingly long waiting list for a heart transplant would die or do not qualify for one of the few donor hearts because of their age and comorbidities. Whereas until recently, INTERMACS profiles 2-4 were used to categorize relatively stringently whether patients were suitable for heart transplantation (“Bridge to Transplant”, BT) or should receive the support system for the rest of their lives (“Destination Therapy”, DT), such therapeutic prognoses are now discouraged due to the nevertheless significant organ shortage with up to two years waiting time. It has been observed that some patients who were initially promised an organ remained on the support system to the end, whereas patients who had been ruled out for transplantation recovered so well on the support system that they could still later undergo heart transplantation [5]. The psychological strain of the family working towards one goal and being surprised by another development also plays a role here. Today’s long-term support systems have no time limit in theory in the absence of complications.
Take-Home Messages
- Mechanical circulatory support is used in patients with refractory cardiovascular failure. A distinction is made between short-term systems with limited duration of use (ECMO, Impella®) and long-term systems (VAD).
- The patient’s INTERMACS profile allows the treating physician to more accurately define the stage of terminal heart failure and thus optimize the timing of support system implantation.
- In everyday clinical practice, acute patients are initially treated with short-term systems. Long-term support systems are rare in practice because of the high all-cause mortality in the acute stage or else the cardiac recovery of some patients in proportion.
Literature:
- Kirklin JK, et al: Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36: 1080-1086.
- Lietz K, Miller L: Patient selection for left-ventricular assist devices. Curr Opin Cardiol 24: 246-251.
- Chatterjee A, et al: The momentum of HeartMate 3: a novel active magnetically levitated centrifugal left ventricular assist device (LVAD). J Thorac Dis 2018; 10(Suppl 15): 1790-1793.
- Rogers D, et al: Intrapericardial left ventricular assist device for advanced heart failure. N Eng J Med 2017; 376: 451-460.
- Teuteberg J, et al: Implant strategies change over time and impact outcomes. JACC Heart Fail 2013; 1(5): 369-378.
CARDIOVASC 2018; 17(6): 13-16