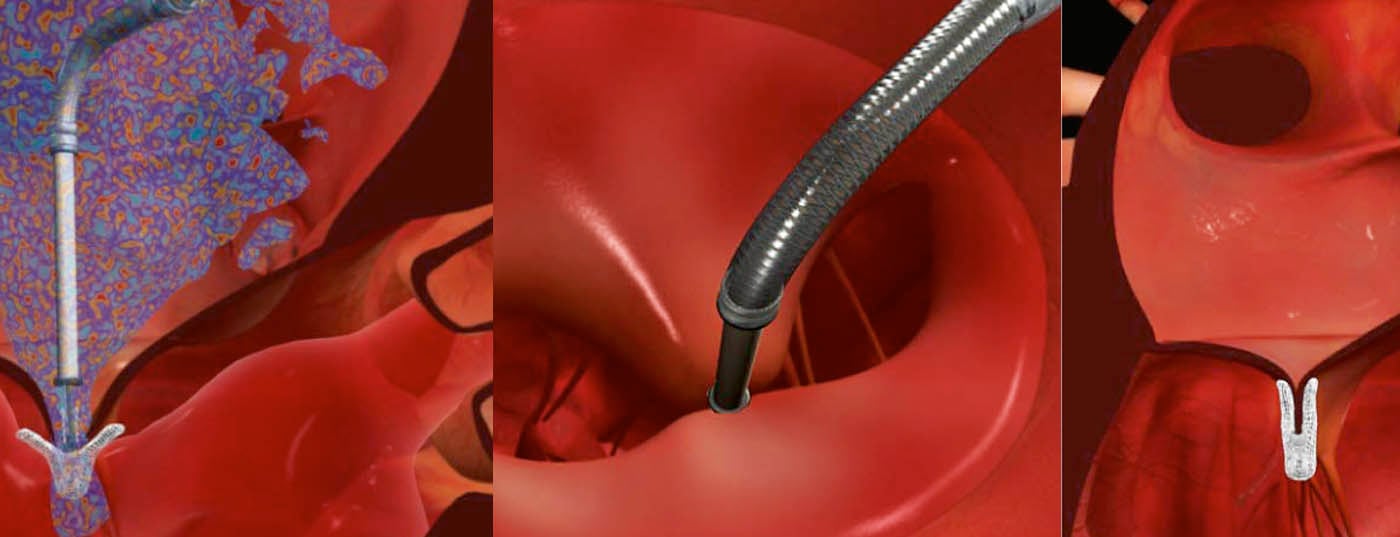
Interventional treatment of mitral regurgitation has become increasingly important in recent years. Important advances in catheter technology as well as imaging now allow us to treat patients who until recently had no options for targeted therapy of mitral regurgitation. These advances will continue in the near future, with a variety of new interventional approaches and techniques currently in preclinical testing. The purpose of this article is to provide an overview of the current status of catheter-based treatment of mitral regurgitation and to provide an outlook on current developments and future possibilities.
Mitral regurgitation is, together with aortic valve stenosis, the most common valve condition in clinical practice. The prevalence of the disease increases with increasing patient age from 0.5% in the under-45 group to over 9% in patients over 75 years of age. The two forms of mitral regurgitation, primary and secondary, have fundamentally different pathomechanisms, which is reflected in the comorbidity and risk profile of the patient groups on the one hand and significantly influences further therapeutic decisions on the other hand. Primary or structural mitral regurgitation is characterized by a defect of the valve apparatus, i.e., the valve leaflets or the chordae, resulting in incomplete closure of the valve in systole. The main causes are degenerative, post-inflammatory, post-rheumatic or idiopathic processes that directly alter the valve structure and thus lead, for example, to prolapse of a valve leaflet or rupture of a tendon thread. Secondary or functional mitral regurgitation is due to geometric changes in the valve holding apparatus and valve annulus in the presence of left ventricular dilatation or pump dysfunction. The underlying myocardial disease causes annulus dilation and displacement of the papillary muscles, making complete valve closure impossible. The mitral valve itself is structurally inconspicuous. The cause is usually ischemic or dilated cardiomyopathy, and patients are characterized by a marked comorbidity profile with the presence of other cardiac pathologies, such as left heart failure, right heart failure, pulmonary hypertension, coronary artery disease, and atrial and ventricular arrhythmias. Patients with secondary valve defects thus generally present with a clinically much more complex overall picture than patients with primary mitral valve defects.
Therapy approaches
The standard therapy for primary mitral regurgitation, given operability, is surgical mitral valve repair, which is indicated in symptomatic patients as well as asymptomatic patients with reduced left ventricular pump function [1]. Compared to conservative drug therapy, this can improve not only the symptoms but also the prognosis of patients with a primary defect. However, in cases of severely reduced pump function and the presence of relevant comorbidities or very high patient age, the risks for surgical intervention increase, so that in clinical practice approximately 50% of all patients with symptomatic mitral regurgitation are not operated on [2]. In patients with secondary insufficiency, who, as described, generally have a high surgical risk a priori, this proportion is even significantly higher and is approximately 90%. These figures ultimately also reflect the recommendations of the guidelines [1], which, due to the surgical risks involved, only consider surgical intervention in the area of the mitral valve to be indicated in cases of secondary mitral regurgitation if surgical revascularization is simultaneously necessary in the presence of coronary artery disease. Since the main pathology of these patients is located in the myocardium, a focus on myocardial unloading or restoration of cardiac output by optimal drug therapy is reasonable, supplemented – if indicated – by cardiac resynchronization therapy or the use of mechanical circulatory support up to heart transplantation.
However, many patients do not qualify for these additional options on a day-to-day basis, so the therapeutic approach usually consists of medication-based symptom control. The presence of mitral valve regurgitation is an independent predictor of morbidity and mortality in patients with both primary and secondary valve defects, making targeted therapeutic approaches important and necessary in this regard as well.
Therefore, there is a need for gentle and low-invasive options for the treatment of mitral regurgitation, justifying the growing use of catheter-based techniques and stimulating research and development in this area.
MitraClip
Since its market introduction in 2008, the MitraClip procedure (Fig. 1) is an interventional technique for the treatment of primary and secondary mitral regurgitation that is becoming increasingly popular.

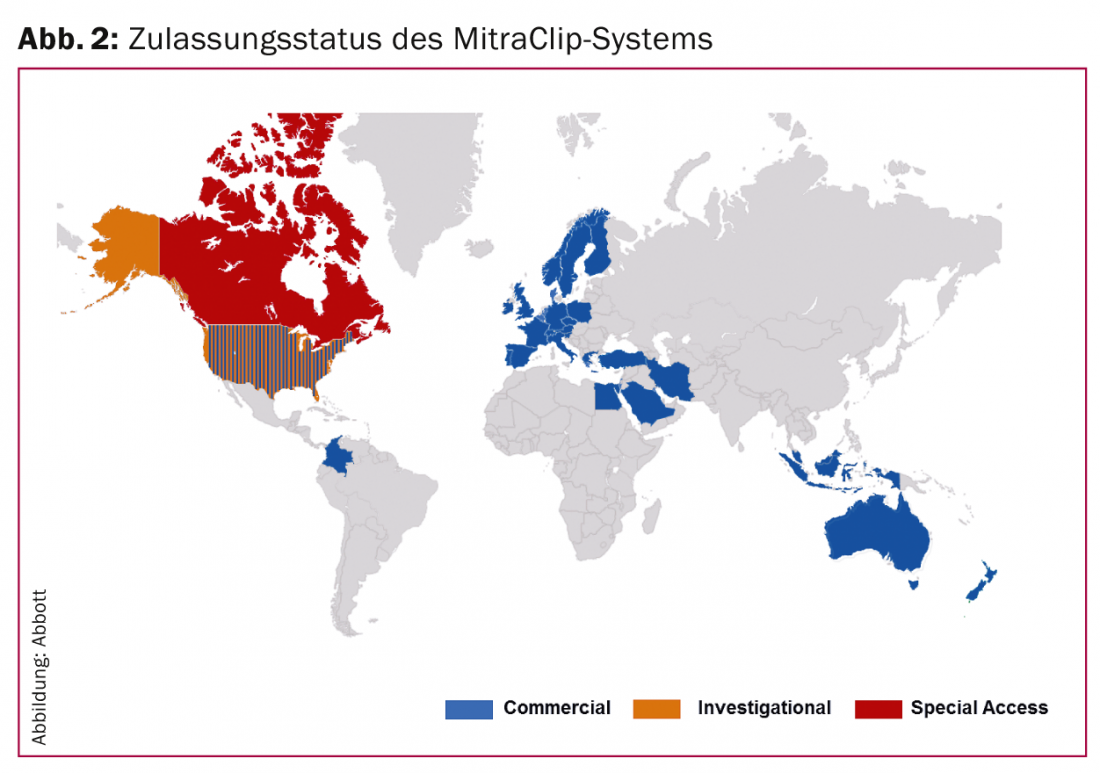
By the end of January 2014, more than 12,000 patients had been treated with this technique in more than 300 centers worldwide (Fig. 2) .
The clip is advanced to the mitral valve via the femoral vein and after transseptal puncture, and ultrasound-guidedly deposited in the area of the insufficiency (Figs. 3-5). The anterior and posterior mitral leaflets are thus permanently connected at this site, which allows restoration of complete valve closure.
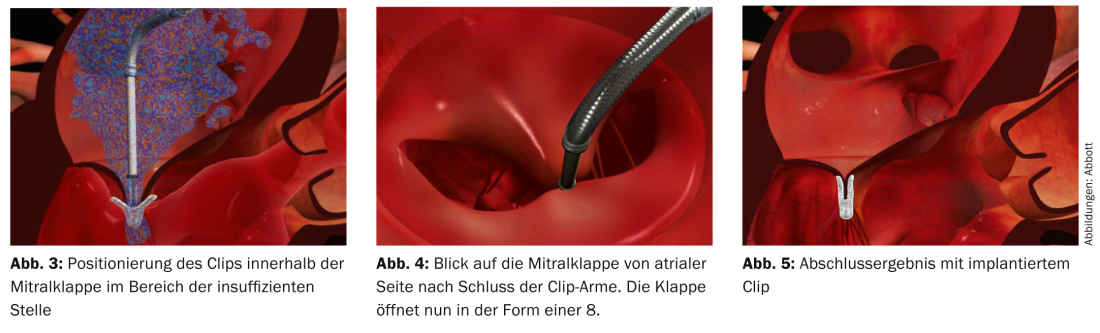
The valve opening area now resembles an 8. The EVEREST II trial, published in 2011, investigated the safety and efficacy of this technique in a randomized comparison with standard surgical therapy in a collective of operable patients including primary and secondary defects [3]. The study showed that the clip procedure provided a significant reduction in insufficiency. The absolute magnitude of reduction is not as high as with surgery, but patients benefited equally clinically with no significant difference in either treatment arm. These results were also confirmed in the long-term course, as demonstrated by the recently published 4-year data of this study [4]. These positive results are also reflected in other published experiences, such as the European postapproval registry ACCESS, the Swiss MitraSwiss registry, the German TRAMI registry, or the Italian GRASP registry, with implantation success rates of 95-100% and a reduction of mitral regurgitation to a maximum of mild to moderate in 80-100% of cases (Table 1).
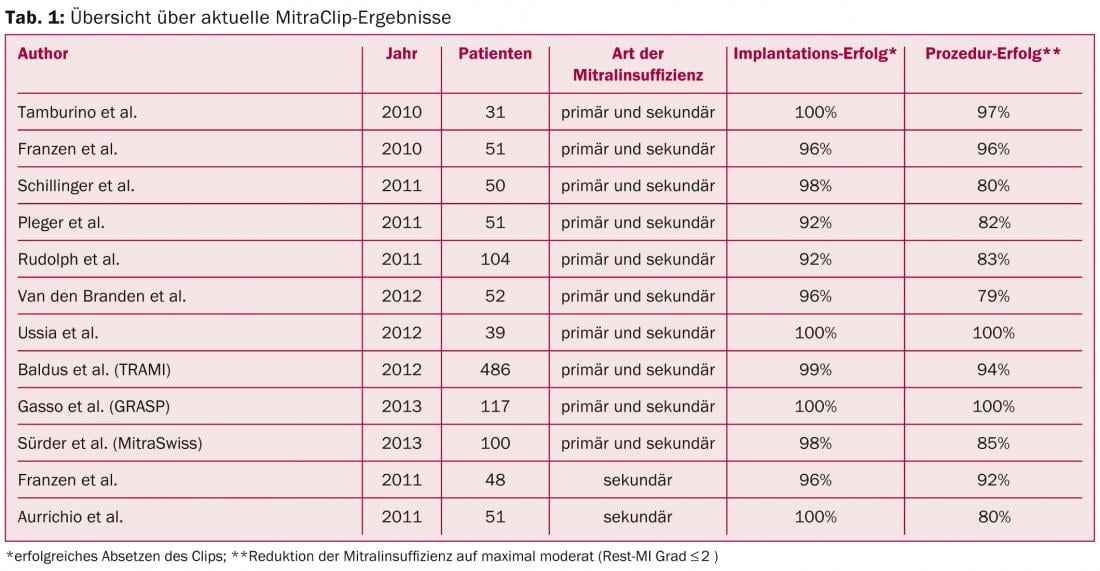
In particular, high-risk clinical groups such as patients with severe heart failure, CRT nonresponders, and inoperable patients with primary valve defect may benefit from the procedure in the presence of severe symptomatic mitral regurgitation. Especially for heart failure patients with concomitant mitral regurgitation, a new therapy alternative is available, which can be used symptom-relieving and potentially prognostically effective. This is the aim of two current randomized trials, the European RESHAPE trial and the American COAPT trial, which compare MitraClip plus optimal drug therapy versus drug therapy alone in this patient group with a similar design with the endpoint of mortality and rehospitalization after 12 months. However, several publications of smaller single-arm studies already suggest that implantation of a MitraClip may lead to symptomatic improvement in these patients.
In 2012, based on these positive data, the MitraClip procedure was included in the European Guideline on Valvular Heart Disease and the European Guideline on Heart Failure Therapy [1, 5], with a recommendation for use in high-risk surgical patients. With the MitraClip technique, we now have a safe, low-cost method to reliably reduce symptomatic mitral regurgitation in high-risk surgical patients, which is associated with clinical improvement in more than 80% of patients.
A limitation of this therapy is the current lack of long-term data. Previous experience with the surgical technique of the Alfieri stitch, which is mimicked interventively by the MitraClip procedure, shows a recurrence of insufficiency over time in a relevant proportion of patients (e.g., due to progression of annulus dilatation and papillary muscle displacement), so that this technique does not play a significant role in everyday surgical practice. Another factor yet to be observed is the immediate postinterventional persistence of moderate mitral regurgitation after MitraClip in approximately one-third of patients. Although a reduction of at least one severity level can be achieved in almost all patients, further investigations are needed to determine to what extent a moderate residual insufficiency affects the long-term course and whether or in which patients a complete reduction of the insufficiency should not be the therapeutic goal. Both limitations mentioned show that despite good and promising results in the acute and mid-term course, further research is needed before, for example, patients with lower surgical risk should be offered this technique. In addition, this creates room for alternative approaches, such as percutaneous valve annuloplasty and percutaneous valve replacement.
Interventional mitral valve annuloplasty
Surgical mitral annuloplasty, i.e., suturing a semi-open or full ring into the mitral annulus to restore valve closure by reducing the annulus area, is the standard surgical treatment for patients with mitral regurgitation. Various interventional approaches attempt to mimic this concept in a minimally invasive catheter-based manner. A basic distinction is made here between two options, indirect and direct catheter annuloplasty.
In indirect procedures, the target of the technique is not the mitral annulus but nearby structures such as the coronary sinus, which is easily accessible by interventional means. The disadvantage of these procedures, of which only the Carillon system has achieved clinical approval to date, is on the one hand the risk of coronary occlusion, since in some cases the coronary arteries cross under the coronary sinus, and on the other hand the not infrequent lack of annulus-parallel anatomical course of the coronary sinus. Systems inserted there therefore exert only a limited influence on the mitral valve and may even have potentially harmful effects.
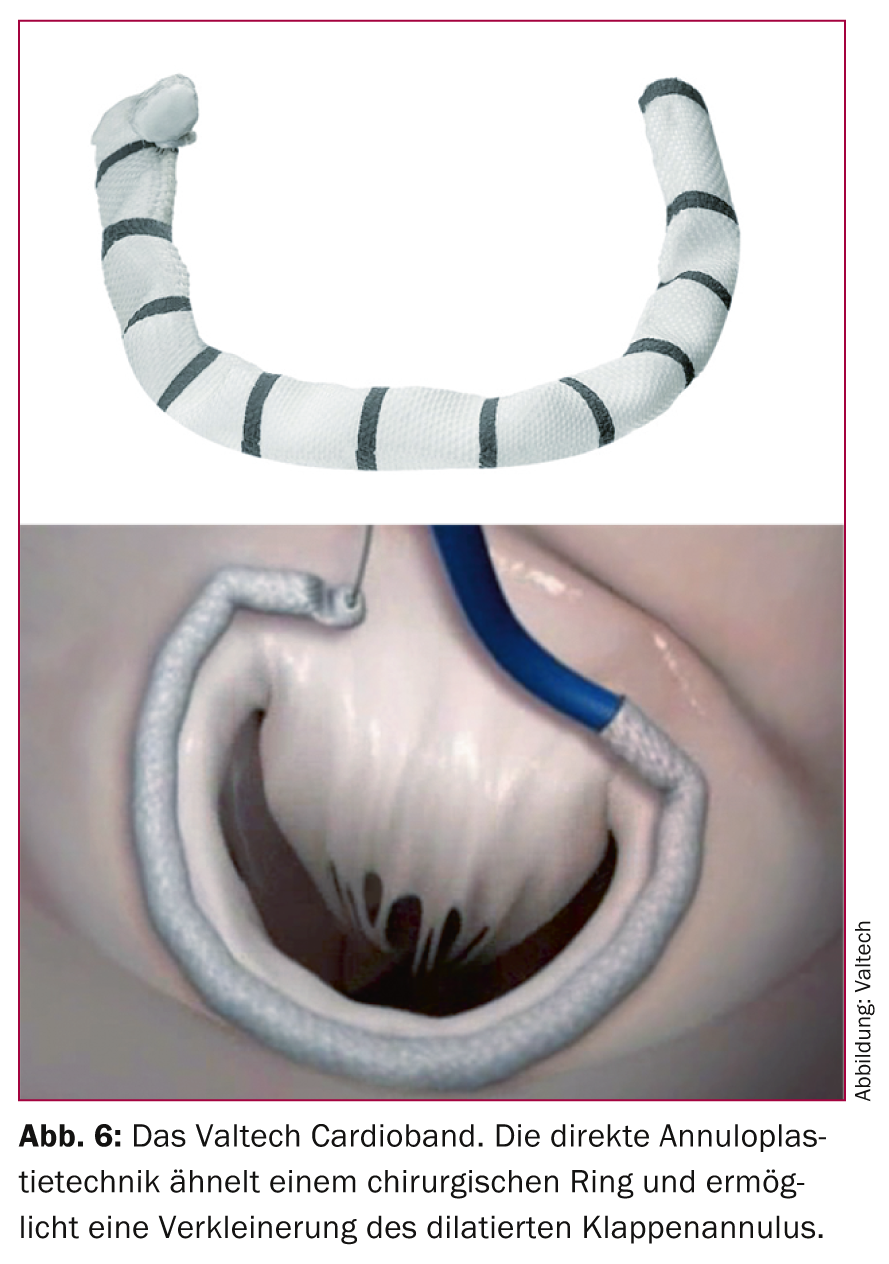
In contrast, direct mitral annuloplasty techniques, which directly target the mitral annulus, are more promising. Several systems are currently under clinical evaluation, including the Mitralign system, the Guided Delivery Systems (GDS) procedure, and the Valtech Cardioband. In particular, the Valtech Cardio Band (Fig. 6), which like the MitraClip can be implanted via femoral venous access, is very close to surgical ring implantation. To date, at the time of writing, a total of 15 patients have been treated with this technique in four European centers, with successful reduction of insufficiency in 14 cases. The results of currently ongoing studies remain to be seen to assess the potential of these approaches. However, the conceptual proximity to the surgical gold standard is certainly a relevant advantage that distinguishes these procedures.
Interventional mitral valve replacement
In contrast to percutaneous aortic valve replacement, the development of catheter-based mitral valve replacement is still in its infancy. Due to the anatomical complexity of the mitral valve apparatus, anchoring and sealing of implanted valve prostheses in particular pose a technical problem. In addition, surgical mitral valve reconstruction has significantly better survival rates compared with surgical valve replacement, especially in patients with primary valve defect, which is why reconstruction is favored in the guidelines. Conceptually, this is fundamentally a disadvantage for the development of interventional mitral replacement techniques. However, especially in the area of secondary insufficiencies, the difference between the two procedures is not very pronounced, particularly when modern replacement techniques are used. Together with the limitations of existing interventional mitral techniques, this is the rationale for developing percutaneous replacement procedures. The CardiaQ system was first used in 2012 for percutaneous implantation of a mitral valve prosthesis to treat an insufficient native mitral valve. Currently, several companies are pursuing this concept and several first-in-man studies are expected to start later this year. It is certainly too early at present to assess these techniques in detail. Potentially, however, this class of interventional techniques will play a role in patients at high risk for traditional surgery who cannot be offered an interventional reconstructive technique with promise.
Conclusions
Interventional techniques for the treatment of mitral regurgitation are becoming increasingly important. The MitraClip system allows safe reduction of mitral regurgitation in patients with primary and secondary regurgitation who are not suitable for surgical intervention due to their risk profile. In particular, patients with heart failure now have a new and less invasive option that significantly expands the existing therapeutic spectrum of optimal drug therapy, cardiac resynchronization therapy, the use of mechanical support systems, and heart transplantation in selected cases. New interventional techniques such as direct valve annuloplasty and percutaneous mitral valve replacement will become established in the coming years and help to address limitations of current interventional procedures.
Literature:
- Vahanian A, et al: Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS): Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012 Oct; 33(19): 2451-2496.
- Mirabel M, et al: What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007 Jun; 28(11): 1358-1365.
- Feldman T, et al: EVEREST II Investigators: Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011 Apr 14; 364(15): 1395-1406.
- Mauri L, et al: EVEREST II Investigators: 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013 Jul 23; 62(4): 317-328.
- McMurray JJ, et al: Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology, Bax JJ, et al: ESC Committee for Practice Guidelines: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012 Aug; 14(8): 803-869.
CARDIOVASC 2014; 13(2): 25-30