To date, molecular testing in non-small cell lung cancer (NSCLC) has been reserved primarily for stage IV patients. However, with the introduction of targeted therapies also in earlier tumor stages, this could soon change – a great opportunity that, however, also poses plenty of challenges for everyday clinical practice.
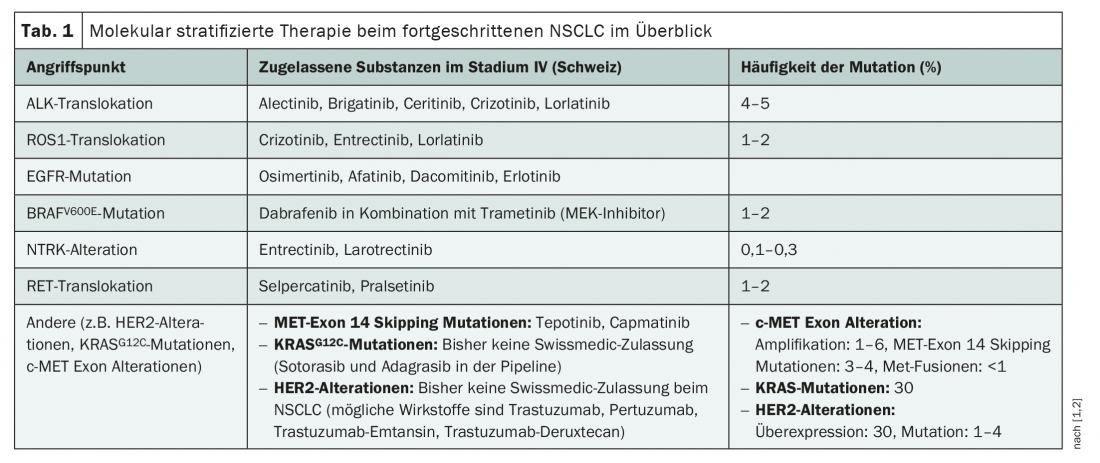
With therapies that specifically target ROS1, ALK, EGFR, BRAF, but also TRK and RET, numerous targeted treatment options already exist for NSCLC (Tab. 1). However, with the exception of the EGFR inhibitor osimertinib, these have so far only been approved in Switzerland for the treatment of locally advanced and metastatic tumors [1]. Accordingly, the diagnosis of therapy-relevant mutations is only recommended in stage IV before the start of first-line therapy [2]. In any case, ALK translocations, BRAFV600E mutations, EGFR exon 18-21 mutations, NTRK fusions, RET translocations, and ROS1 translocations should be included [2] – because there are approved therapeutic approaches for these alterations. However, targeted therapy in NSCLC is undergoing rapid change, and with it molecular testing. On the one hand, this could be expanded in the near future with additional targets, as there are some new compounds in the pipeline. On the other hand, targeted oncologics are increasingly being tested for neoadjuvant and adjuvant use in earlier tumor stages – with success, as evidenced by the recent approval of osimertinib in the adjuvant setting. The logical consequence: molecular tumor classification could already be useful for stage I-III tumors.
Further points of attack in sight
Currently, in addition to the well-known Driver mutations, numerous others are in the spotlight. For example, various targeted agents directed against HER2 or KRAS, among others, could be approved in the near future. Only in April and June of this year, respectively, the highly specific MET inhibitors capmatinib and tepotinib for the treatment of metastatic NSCLC with a MET tyrosine kinase receptor exon 14 skipping mutation entered the Swiss market [1]. This genetic alteration still affects 3-4% of NSCLC patients and is associated with an unfavorable prognosis [2].
In the pivotal VISION trial, which included 152 mostly pretreated patients with MET exon 14 skipping mutation, tepotinib resulted in an objective response rate of 44.7% with a median progression-free survival (PFS) of 8.9 months [1]. For capmatinib, the objective response rate in the GEOMETRY mono-1 pivotal trial was as high as 67.9%, and the median PFS was 9.69 months. Further data on overall survival and comparison of efficacy with standard treatment using immunochemotherapy remain to be seen. What is already certain is that testing for MET exon 14 skipping mutations will likely expand the diagnostic standard in the future.
KRAS mutations and HER2 aberrations may also soon be added to molecular diagnostics in NSCLC. Although no specific therapies have yet been approved in Switzerland for these targets, there is a lot going on in the pipeline. For example, soterasib and adagrasib were the first KRASG12C inhibitors to be developed and are currently in clinical trials. EU approval for soterasib has been filed and is expected later this year, and the U.S. Food and Drug Administration (FDA) has already granted preliminary approval for the therapy of pretreated patients with KRASG12C mutation [3,4]. About 13% of NSCLC patients could benefit from a market launch – that’s how common the KRASG12C mutation is [3]. In the phase II CodeBreak 100 trial, 124 pretreated patients with KRASG12C mutation showed a 37.1% response rate to sororasib with a median PFS of 6.8 months and a median OS of 12.5 months [5]. In contrast to KRAS, agents such as trastuzumab, pertuzumab, and the antibody-drug conjugates trastuzumab emtansine and trastuzumab deruxtecan already exist for targeting HER2. While their use is being investigated in some clinical trials in NSCLC, to date there have been no direct comparisons versus immunochemotherapy and no Swissmedic approval in NSCLC [1,2].
Adjuvant and neoadjuvant therapy in transition
In addition to expanding the molecular characterization of NSCLC, its importance in earlier tumor stages may also increase. Osimertinib makes the start. Thus, this year the EGFR inhibitor was approved for the adjuvant treatment of patients with non-squamous NSCLC with EGFR exon 19 deletions or exon 21 (L858R) substitution mutations after complete tumor resection in Switzerland [1]. This was based on the ADAURA trial, in which adjuvant therapy over three years in stages II and IIIA after R0 resection resulted in a significant improvement in disease-free survival compared with placebo (HR 0.17; p<0.001) [2]. Most study participants also received adjuvant chemotherapy. The new option of adjuvant targeted treatment means hope for those NSCLC sufferers with EGFR mutation – and requires appropriate patient selection.
Approval of additional targeted agents in the adjuvant and neoadjuvant settings is likely. Thus, fair and uniform molecular testing strategies become necessary. While many guidelines do not yet include a recommendation for molecular analysis in tumor stages I-III, the guidelines of the National Comprehensive Cancer Networks (NCCN) and the Asian Thoracic Oncology Research Group, the Canadian Consensus Recommendations, the Chinese guidelines for diagnosis and treatment of primary lung cancer, and the Society for Translational Medicine consensus recommended at least EGFR testing. This makes sense not only in view of the new therapeutic option, but also because EGFR mutations are so-called truncal mutations: They are present from the beginning and their prevalence hardly changes from stage to stage. In addition to EGFR inhibitors, ALK inhibitors such as alectinib are currently being tested in neoadjuvant, adjuvant, and maintenance therapy of NSCLC. The guidelines will probably always lag somewhat behind these developments – and their implementation in everyday clinical practice will in turn take a while. This makes it all the more important to assess each patient individually and, in view of the numerous new possibilities, to think outside the box of entrenched structures.
Source: Aggarwal C, et al.: Molecular testing in stage I-III non-small cell lung cancer: Approaches and challenges. Lung Cancer. 2021; 162: 42-53.
Literature:
- Swissmedic drug information: www.swissmedicinfo.ch (last accessed 11/25/2021).
- Griesinger F, et al: Onkopedia Guidelines: Lung cancer, non-small cell (NSCLC). As of July 2021. www.onkopedia.com/de/onkopedia/guidelines/lungenkarzinom-nicht-kleinzellig-nsclc/@@guideline/html/index.html (last accessed Nov. 25, 2021).
- Whitlock Burton K: FDA approves soterasib as first targeted therapy for NSCLC with KRAS G12C mutations. 03.06.2021. www.univadis.de (last accessed 11/25/2021).
- DGHO: NUB Sample Request Sotorasib 2021/2022. www.dgho.de (last accessed 11/25/2021).
- Skoulidis F, et al: Sotorasib for Lung Cancers with KRAS p.G12C Mutation. New England Journal of Medicine. 2021; 384(25): 2371-2381.
InFo ONcOLOGy & heMATOLOGy