Narrow complex tachycardia is a common clinical presentation. Reentry, in particular, provides persistent tachycardia. The prerequisite for the development of reentry tachycardia is a substrate with two distinct conduction pathways that have different conduction velocities and refractory periods. Supraventricular tachycardias are not associated with structural heart disease in most cases.
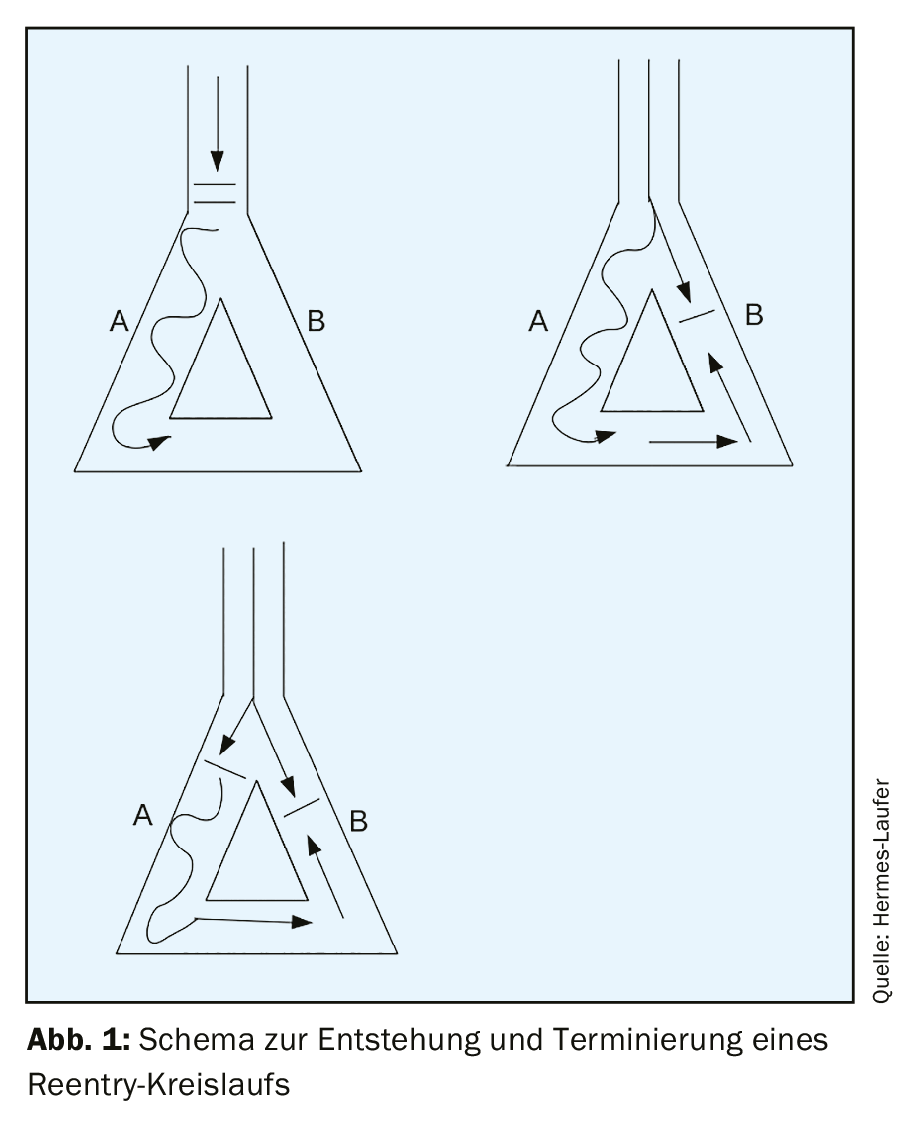
Regular narrow complex tachycardia is a common clinical presentation with a prevalence of 2.25/1000 patients [1]. The three underlying mechanisms of tachycardia in general are automaticity, reentry, and triggered activity. By far the most common of these three mechanisms in sustained tachycardia is reentry. Automaticity occurs focally and is rare (less than 10% of all tachycardias). Triggered activity is a disturbance of repolarization – where post-depolarizations occur during phase III or phase IV of the action potential and as soon as the threshold is reached a renewed action potential occurs and thus tachycardia. Supraventricular reentry tachycardias are divided into AV nodal reentry tachycardias, AV reentry tachycardias, and focal atrial reentry tachycardias with a frequency distribution of 60%, 30%, and 10%, respectively [2]. The prerequisite for the development of reentry tachycardia is a substrate with two distinct conduction pathways that have different conduction velocities and refractory periods. If a trigger, such as an extrasystole, occurs at the same time that the conduction pathway with the longer refractory period is still refractory, but the pathway with the shorter refractory period has already recovered, a unidirectional block occurs initially and can then lead to a circling excitation if the excitation arrives at the time when the second conduction pathway is able to conduct again (excitable gap). (Fig. 1). Supraventricular tachycardias are not associated with structural heart disease in most cases.
Differential diagnosis
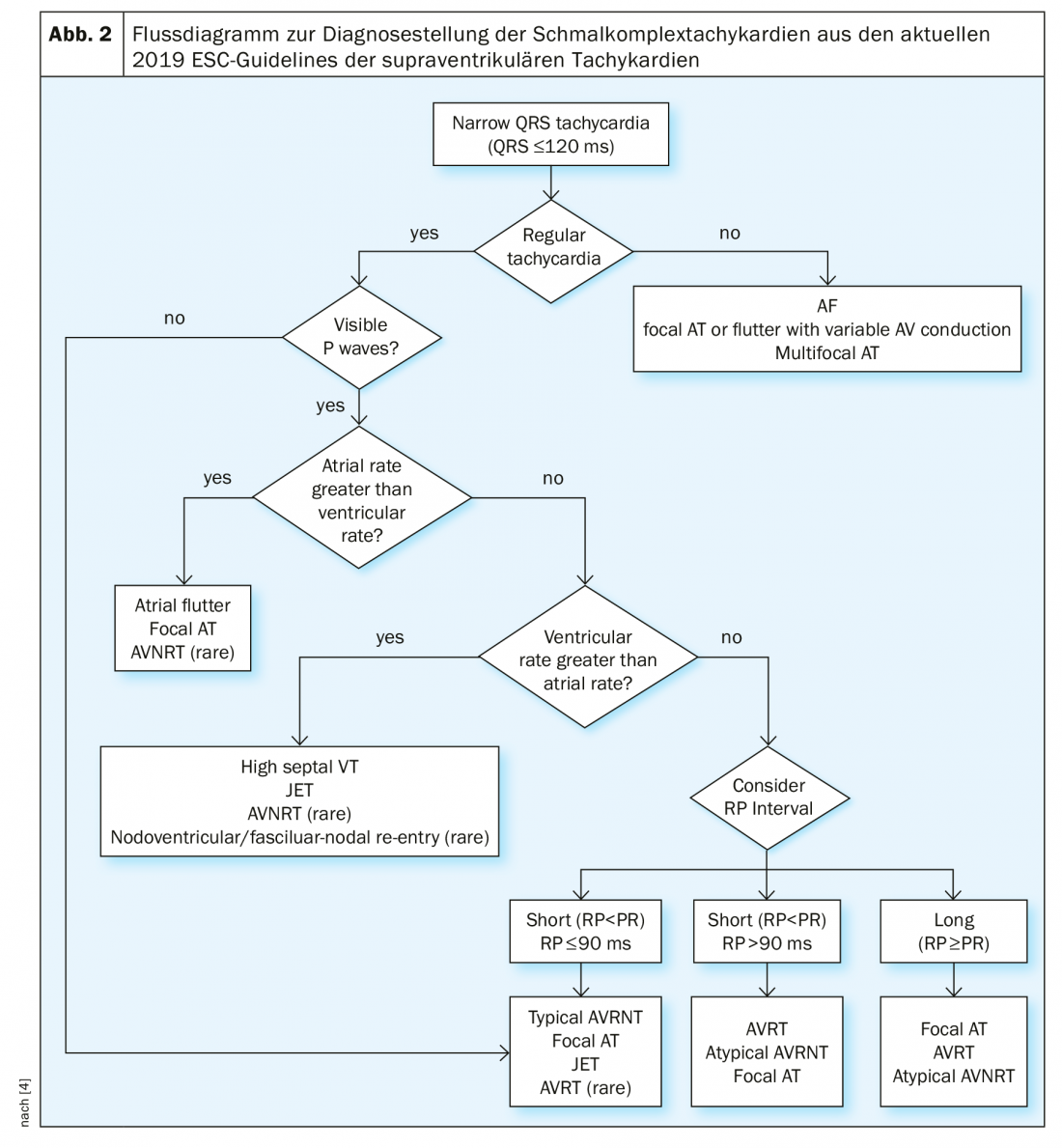
In cases of unexplained narrow complex tachycardia with a QRS duration <120 ms, the patient should first be assessed for hemodynamic stability and a 12-lead ECG should be obtained whenever possible. Monitor surveillance is recommended, as well as laboratory analysis including blood counts, electrolytes, renal function, and thyroid function. In the 12-lead, the tachycardia must be checked for regularity and the presence of P waves. Irregular ventricular rate is usually atrial fibrillation, atrial flutter with variable conduction, or focal atrial tachycardia. In particular, the onset and termination of tachycardia are of great importance, e.g., with the question of sudden or progressive onset and termination. If P waves are present, verify that a P wave is associated with each QRS complex, that the PR distance is greater than the RP distance (PR>RP or RP>PR), and that the atrial rate is greater than, less than, or equal to the ventricular rate. An overview of the differential diagnosis of narrow complex tachycardia is provided by the flowchart from the current ESC guidelines (Fig. 2) . In unclear cases and hemodynamic stability, a diagnostic vagal maneuver and/or intravenous adenosine administration should be considered. Thus, in atrial flutter, the flutter waves manifest and AVNRT or AVRT is terminated in most cases by adequate administration of adenosine. If adenosine has no effect on narrow complex tachycardia, it was either injected at an inadequate dosage and/or mode of administration (injected too slowly or without NaCl flush) or, in rare cases, high septal ventricular tachycardia.
AVNRT
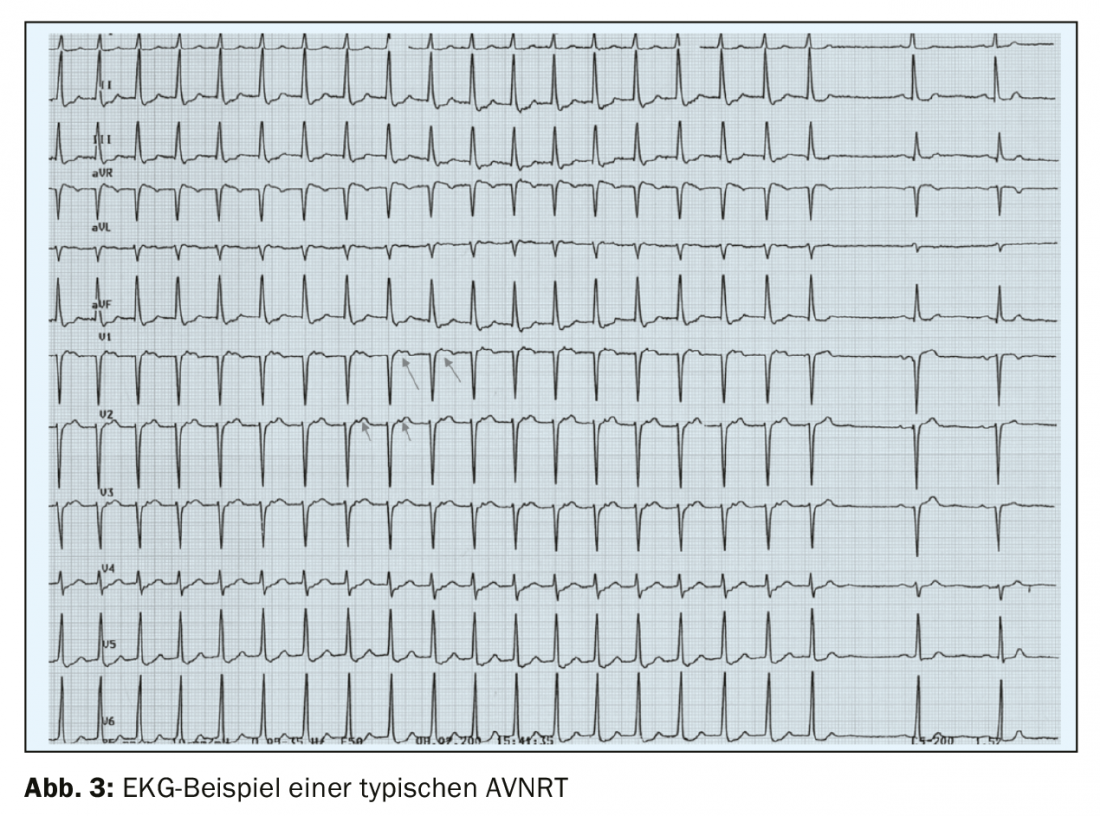
The most common form of paroxysmal supraventricular tachycardia – AV nodal reentry tachycardia (AVNRT) is characterized by a sudden onset and an equally sudden termination. Approximately 95% of cases are typical AVNRT of the slow-fast type, with antegrade conduction via the slow conduction pathway and retrograde conduction via the fast conduction pathway. In the history it must be asked whether there is an increased diuresis during/after the tachycardia as well as a feeling of “knocking in the throat” (frog sign), which are typical symptoms of AVNRT due to the simultaneous contraction of the atrium and ventricle with closed valves and associated ANP release. Surface ECG shows regular narrow complex tachycardia (except in the presence of preexisting bundle branch block) without visible P waves or with retrograde P waves with a very short RP interval of less than 70 ms (Fig. 3) [4]. In atypical AVNRT (circa 5% of AVNRTs) of the fast-slow or slow-slow type, retrograde P waves are often visible before the subsequent QRS complex because retrograde excitation travels via the slow pathway.
AVNRT may manifest in childhood or early adulthood as well as in the fourth or fifth decade of life or even later [3,4]. Dual conduction physiology is innate, but the likelihood of occurrence of AVNRT modulates with age, partly because of variably frequent extrasystoles and partly because of age-related structural modifications in the properties of the two conduction pathways, which may also lead to late manifestation of AVNRT [3]. Other factors that may influence the likelihood of manifestation of AVNRT through clustered triggers include hormonal factors, such as in hyperthyroidism, and excessive consumption of caffeine and alcohol.
AVRT
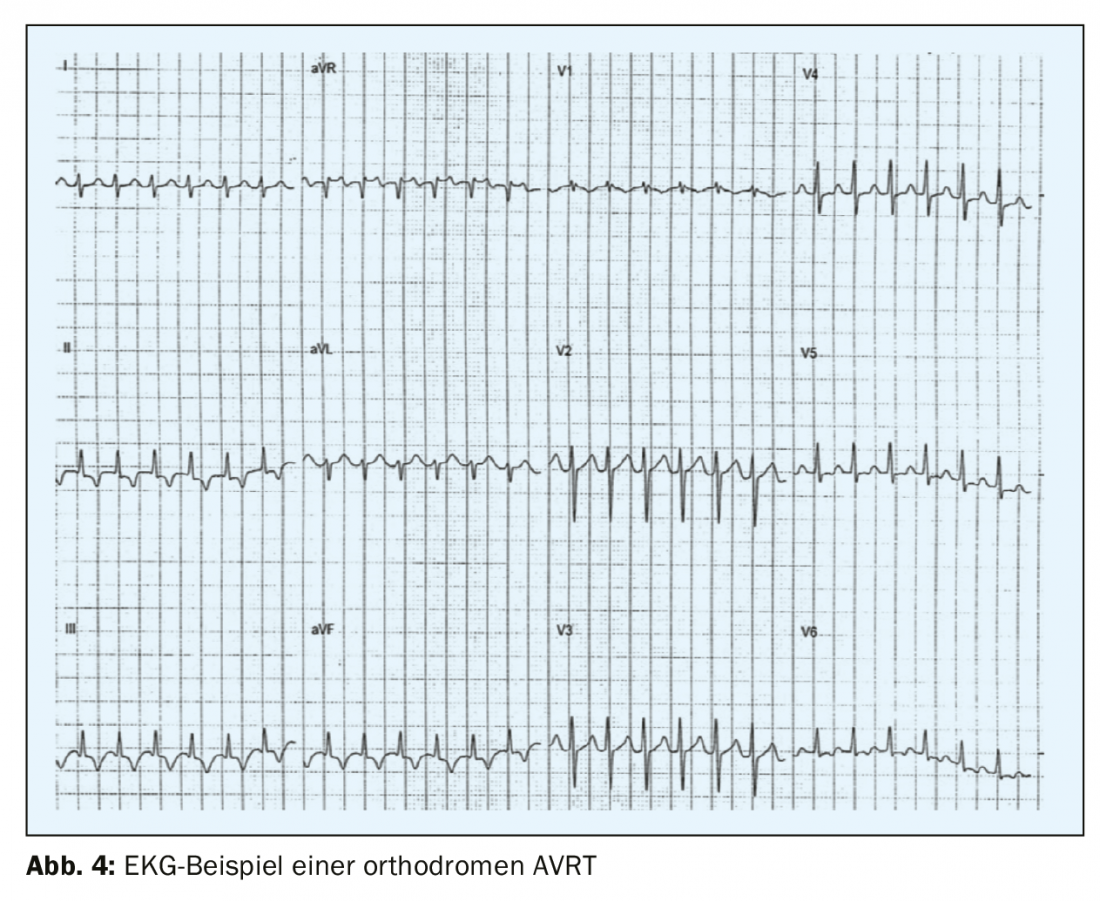
The second most common form of paroxysmal supraventricular tachycardia – AVRT – results from an accessory pathway between the atrium and ventricle, which is outside the specific conduction system. The pre-excitation syndrome – or Wolff-Parkinson-White syndrome was first described in the early 1930s by the three cardiologists who gave it its name, Louis Wolff, John Parkinson and Paul Dudley White, and almost simultaneously by Max Holzmann in Zurich. Accessory pathways along the mitral valve are most commonly localized to the left free wall (circa 60-70% of accessory pathways), followed by septally localized pathways at the mitral or tricuspid annulus (circa 25%), and only 15% are localized to the RV free wall [5]. Some patients (<12%) have multiple accessory pathways, which should be considered, for example, in patients with Ebstein’s anomaly [6]. In preexcitation syndrome, a manifest accessory pathway is present, frequent arrhythmias occur, and a typical picture of preexcitation is seen on the ECG with the typical delta wave, a flattened up- or downstroke of the QRS complex, and a widened QRS complex of more than 120 ms. In most cases, patients with WPW syndrome have a structurally normal heart and are in childhood or adolescence at initial manifestation. Men are more frequently affected than women. In orthodromic AVRT (>90% of AVRTs and 20-30% of all sustained supraventricular tachycardias), the circuit first runs from the atrium to the ventricle via the normal conduction system and back from the ventricle to the atrium via the accessory pathway. The seizure ECG shows regular narrow complex tachycardia with a rate of mostly 160-220/min with retrograde P waves with an RP interval of >70 ms (RP<PR) (Fig. 4) .
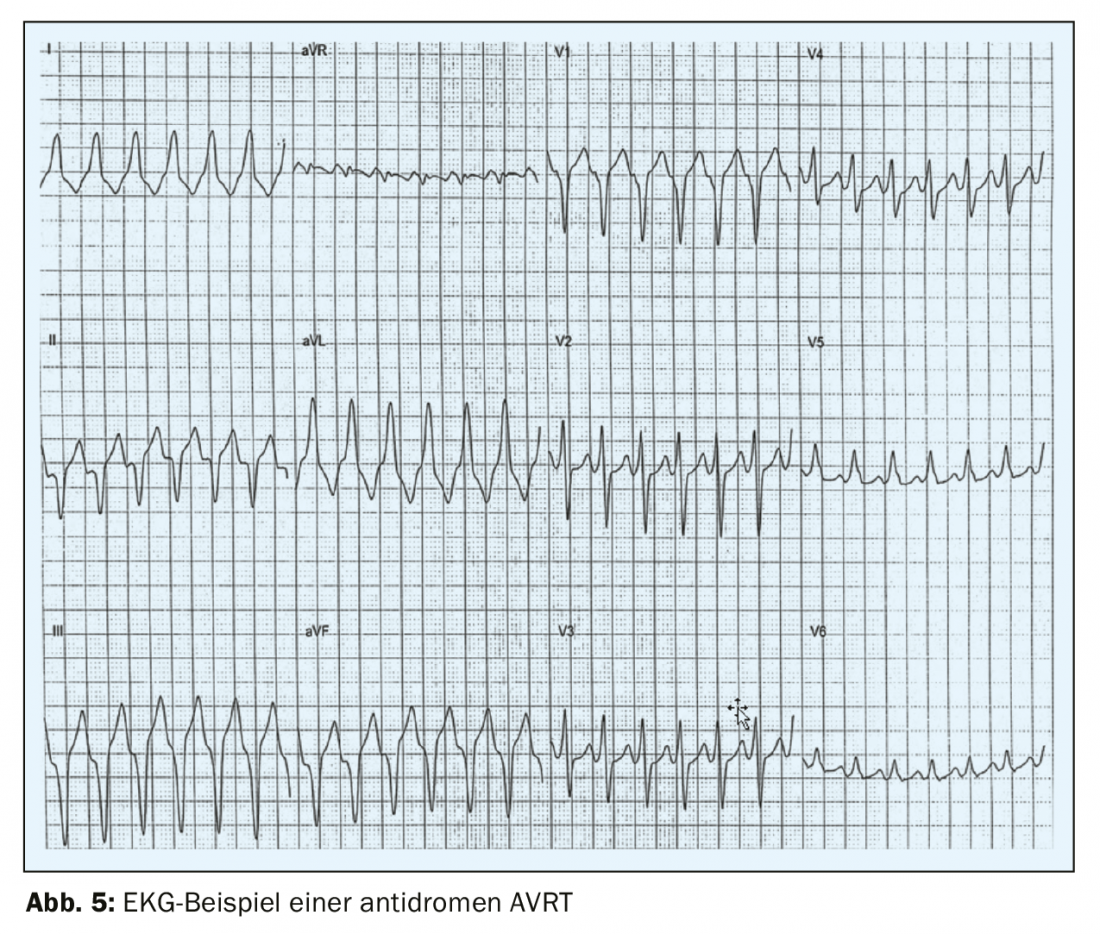
The much rarer antidromic AVRT occurs in only 3-8% of WPW patients [7] with excitation initially running from the atrium to the ventricle via the accessory pathway and back via the AV node. The surface ECG shows a wide QRS complex in this case, which is why antidromic AVRT is difficult to distinguish from ventricular tachycardia (Fig. 5) . Paroxysmal atrial fibrillation occurs in 50% of patients with WPW syndrome [8,4]which can be life-threatening and degenerate into ventricular fibrillation if conducted via the accessory pathway and at a fast ventricular rate. On the surface ECG, this arrhythmia is recognized by a fast, irregular rate and a broad QRS complex with pre-excitation [9] (FBI – fast, broad, irregular). For this reason, it is crucial to always clarify rhythmologically even incidental findings of a preexcitation ECG in asymptomatic patients and to treat them in most cases. In so-called concealed WPW, there is an accessory pathway that conducts only retrogradely – usually at the free LV wall, but the preexcitation is not visible on the surface ECG and the initial manifestation is orthodromic AVRT. Because the accessory pathway cannot conduct anterograde in this case, patients with a concealed WPW are not at increased risk for sudden cardiac death.
Atrial tachycardia
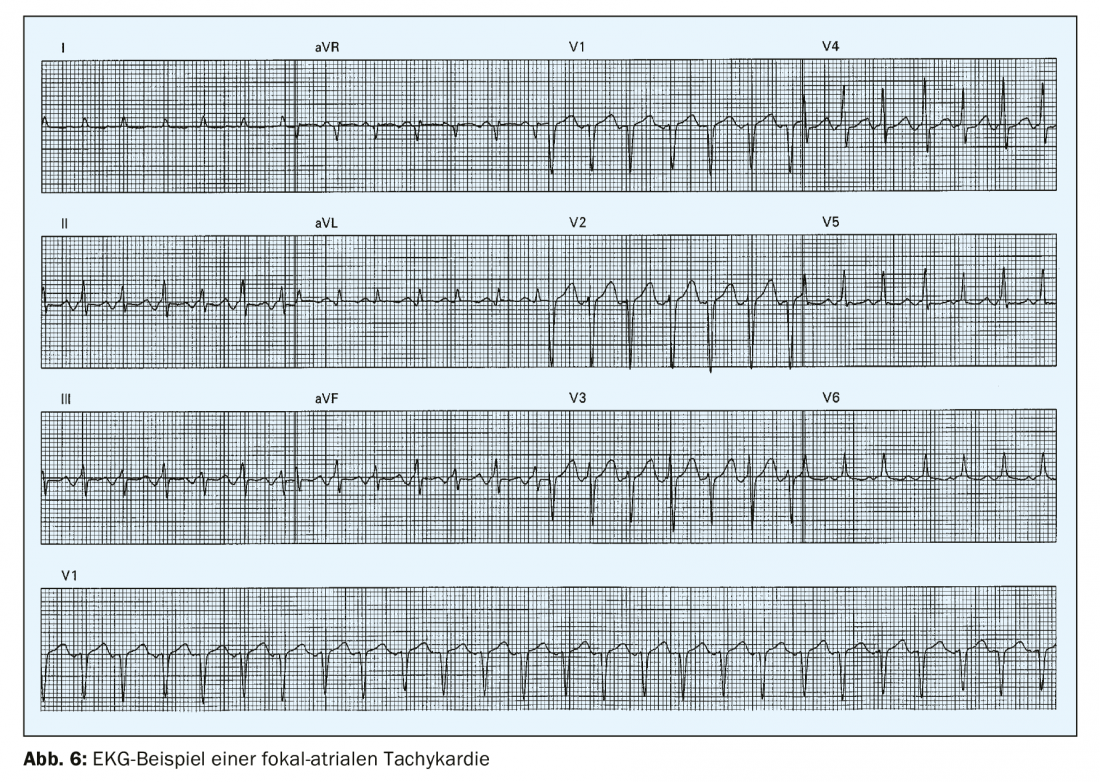
Focal atrial tachycardia is an organized atrial rhythm >100/min that originates outside the sinus node (Fig. 6) . It is rare with a prevalence of 0.34% in young adults [4] and accounts for less than 10% of all SVTs. In the surface ECG, monomorphic P waves with a stable cycle length are found. The triggering mechanism can be, on the one hand, a micro-reentry or an automaticity. Frequently, focal atrial tachycardia originates in the area of veno-atrial junctions, e.g., at the coronary sinus, in the area of the crista terminalis, or the mitral or tricuspid annulus. The configuration of the P wave on the 12-lead ECG may provide information about the origin of the focal atrial tachycardia.
Atrial Flutter
In atrial flutter, one must distinguish between typical isthmus-dependent atrial flutter (90%) with either counterclockwise or clockwise macroreentry around the cavotricuspid isthmus (tricuspid annulus) and atypical flutter. In typical atrial flutter, a further distinction is made between type I with counter-clockwise macroreentry around the cavotriscupid isthmus and type II with clockwise macroreentry. The respective ECGs in type I and type II typical atrial flutter can be very different – for example, the typical “sawtooth waves” occur in type I flutter and may be completely absent in type II flutter, where they are often hidden in the QRS complex and the T waves. Atypical atrial flutter can originate in either the right or left atrium and is commonly observed in preoperated patients, patients with congenital heart defects, or after interventions such as atrial fibrillation ablations in which there is an appropriate substrate around which the flutter circuit rotates. On the surface ECG, typical flutter is seen to have sawtooth-like flutter waves on the ECG, usually with a frequency of around 300/min and presenting negatively in the inferior leads and positively in V1 in the case of typical counter-clockwise isthmus-dependent atrial flutter. AV transition is often 2:1, but may be 3-4:1, 1:1, or variable. Therefore, in case of a regular SVT with a frequency around 150/min, atrial flutter should always be considered as a differential diagnosis, regardless of whether flutter waves are visible on the surface ECG or not. The AV node physiologically goes into a 2:1 or higher block in atrial flutter, due to its refractory period and decremental conduction property. Clinically, atrial flutter is often associated with atrial fibrillation and patients should be screened for this.
A discussion of atrial fibrillation is beyond the scope of this article, except to mention that irregular tachycardia with absolute arrhythmia without clear P waves is atrial fibrillation for which ECG documentation must be sought for further management.
Management
Management of AVNRT: In acute therapy, synchronized cardioversion should be performed in hemodynamically unstable patients, which is extremely rarely necessary in practice (class IB indication). Vagal maneuvers, such as the Valsalva maneuver with abdominal press, unilateral carotid sinus massage, or rapid drinking of cold water, can often terminate reentry tachycardia (Class IB indication). Carotid sinus massage should always be preceded by auscultation to identify carotid stenosis and should generally be performed with restraint in elderly patients with atherosclerosis. If vagal maneuvers fail, the patient is stable, and there are no contraindications, 6-18 mg adenosine should be administered i.v. briskly and with NaCl flush (class IB indication). The application of antiarrhythmic drugs such as beta-blockers iv or verapamil or diltiazem is included as a therapeutic option in the current guidelines (class IIa indication), but may lead to hypotension and transient AV block and is nowadays rarely necessary in practice. The gold standard for long-term therapy of both typical and atypical AVNRT is catheter ablation, which leads to cure in 97% of cases and serious complications [10] such as AV block and pacemaker dependency in only 0.3%. The complication rate depends on the experience of the electrophysiologists. There is no age limit for catheter ablation; seniors of advanced age with comorbidities should also undergo catherablation.
Management of AVRT: In acute therapy, synchronized cardioversion should be performed in hemodynamically unstable patients (class IB indication). Vagal maneuvers, like AVNRT, are also indicated and may terminate tachycardia (class IB indication). Drug therapy must be differentiated between orthodromic and antidromic AVRT.
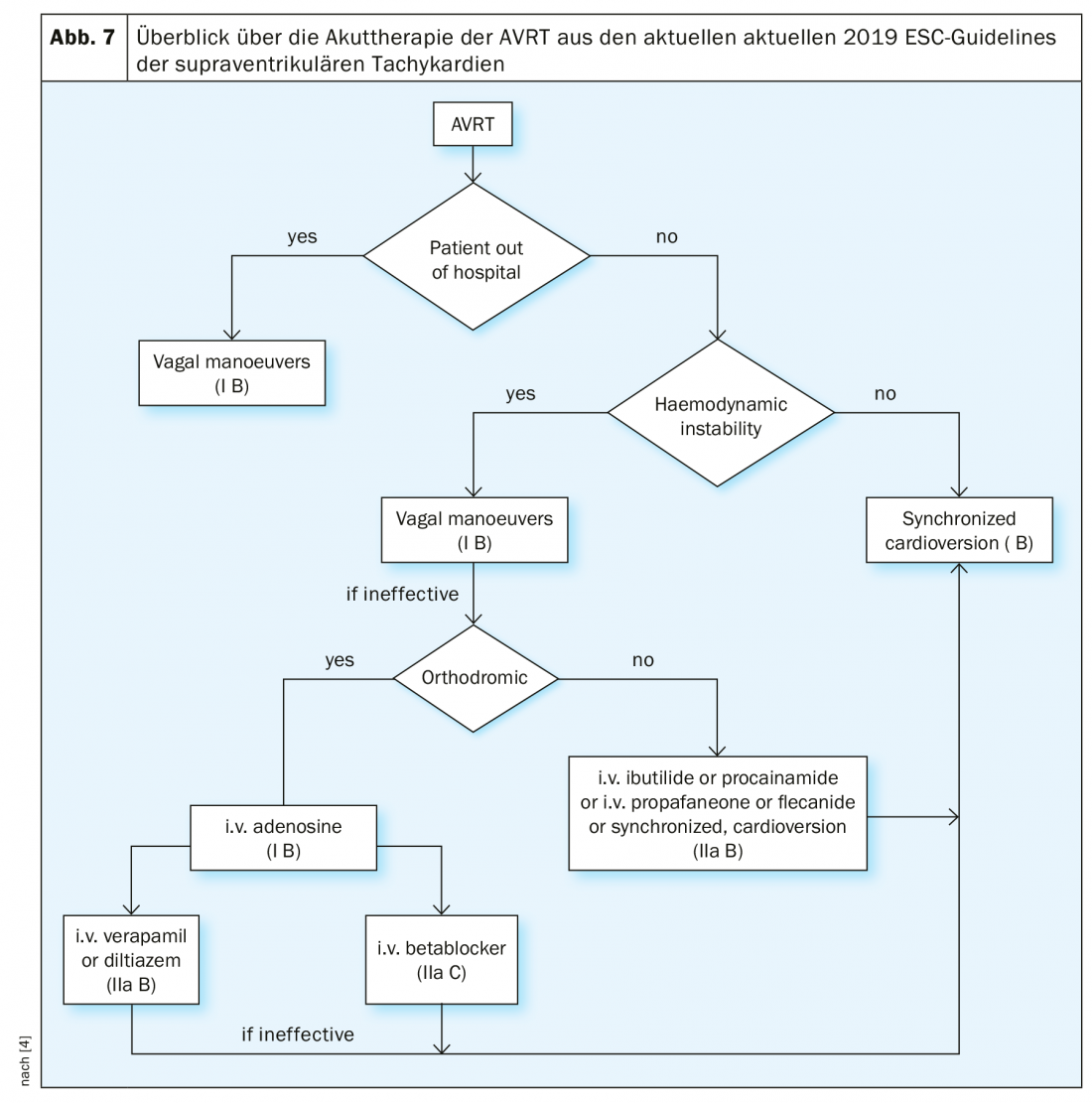
In orthodromic AVRT, adenosine 6-18 mg should be administered iv (class IB indication), which is even more efficient in AVRT than in AVNRT. Negative chronotropic drugs should be avoided in antidromic AVRT. Therapeutic options besides synchronized cardioversion in this case are flecainide, procainamide, or propafenone-as in atrial fibrillation with preexcitation. The flowchart from the current SVT guidelines provides an overview of acute therapy for AVRT (Fig. 7).
Following acute treatment, the gold standard, as with AVNRT, is ablation of the accessory pathway. The complication rate of ablation depends on the location of the accessory pathway; major complications include complete AV block (0.17-2.7%) and pericardial tamponade (0.1-1.1%) [4]. Left accessory pathways can be ablated using either a transseptal or retrograde aortic approach. If catheter ablation is not desired by the patient, beta-blockers, diltiazem, or verapamil can be used in concealed WPW, and class IC antiarrhythmics can be used in manifest, “overt” WPW. In overt WPW, verapamil-type calcium antagonists (and digitalis drugs) are contraindicated because they slow conduction across the AV node, favoring rapid conduction across the accessory pathway and thus a threateningly rapid ventricular rate.
Management of atrial flutter: drug frequency control is not always easy to achieve in atrial flutter; combinations of negative chronotropic drugs (beta-blockers, calcium antagonists, digoxin) may be used with caution. Anticoagulation should be initiated according to the same criteria as for AF (CHA2DS2-VASc score ≥1 point). It is not uncommon for synchronized cardioversion to be necessary in acute therapy because medical rate control is insufficient. Amiodarone can also be used for rate control, but can achieve rhythm control in only 29% of cases [11], so the primary strategy for rhythm control in atrial flutter is synchronized cardioversion. Cardioversion of atrial flutter requires less energy than cardioverting atrial fibrillation and is more efficient. In patients with a pacemaker and an existing atrial lead, atrial overstimulation can lead to termination of flutter. Adenosine should be used only to confirm the diagnosis and unmask flutter waves in the presence of an unclear ECG and may provoke atrial fibrillation. The gold standard of long-term therapy is catheter ablation, which leads to 90% freedom from recurrence in isthmus-dependent atrial flutter. Ablation of atypical atrial flutter is more difficult because multiple electrical circuits around individual substrates often must be mapped and ablated. In atrial fibrillation, the aim should be to achieve rhythm control that is appropriate to the patient’s overall situation, if possible. It is imperative that anticoagulation guidelines be followed for all rhythm and rate control measures in atrial flutter and atrial fibrillation.
Management of focal atrial tachycardia: In acute therapy, beta-blockers or calcium antagonists can be used to slow the ventricular rate [4]. Adenosine can lead to termination or even bradycardia in about 50% of cases and therefore does not always help in diagnosis and therapy [12]. In symptomatic patients with recurrent focal atrial tachycardia, catheter ablation should be performed.
Take-Home Messages
- Synchronized cardioversion should be performed in hemodynamically unstable patients, regardless of the form of SVT.
- Documentation of tachycardia by 12-lead ECG is of great importance for further management and should always be sought.
- Vagal maneuvers and adenosine (6-18 mg as an i.v. bolus) are indicated with hemodynamic stability for both confirming the diagnosis and for
- Acute therapy provided there are no contraindications.
- Catheter ablation is a safe, effective long-term therapy for AVNRT, AVRT, and typical atrial flutter with high rates of freedom from recurrence and low rates of complications at specialized centers.
- In atrial tachycardia, therapy is initially medicinal, but in symptomatic patients with recurrences, the indication for catheter ablation should also be considered.
Literature:
- Delacretaz E: Supraventricular tachycardia. New England Journal of Medicine, 2006; 354(10): 1039-1051.
- Wellens HJ: Electrophysiology, 25 years of insights into the mechanisms of supraventricular arrhythmias: NASPE HISTORY SERIES. 2003; 26(9): 1916-1922.
- Pentinga ML, et al: Late onset atrioventricular nodal tachycardia. 1993; 38(3): 293-298.
- Brugada J, et al: 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). 2019.
- Jackman WM, et al: Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. 1991; 324(23): 1605-1611.
- Cappato R, et al: Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein’s anomaly. 1996; 94(3): 376-383.
- Brembilla-Perrot B, et al: Incidence and prognostic significance of spontaneous and inducible antidromic tachycardia. 2013; 15(6): 871-876.
- Gemma LW, et al: Development of rapid preexcited ventricular response to atrial fibrillation in a patient with intermittent preexcitation. 2013; 24(3): 347-350.
- Etheridge SP, et al: Life-threatening event risk in children with Wolff-Parkinson-White syndrome: a multicenter international study. 2018; 4(4): 433-444.
- Spector P, et al: Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. 2009; 104(5): 671-677.
- Kafkas NV, et al: Conversion efficacy of intravenous ibutilide compared with intravenous amiodarone in patients with recent-onset atrial fibrillation and atrial flutter. 2007; 118(3): 321-325.
- Eidher U, et al: Efficacy and safety of ibutilide for the conversion of monomorphic atrial tachycardia. 2006; 29(4): 358-362.
CARDIOVASC 2020; 19(2): 6-11