In a post-hoc analysis of large randomized placebo-controlled trials, HbA1c and body weight and blood pressure were examined as mediator variables regarding the renal protective effects of the GLP-1 receptor agonists semaglutide and liraglutide in type 2 diabetes. The results were presented at this year’s EASD Virtual Meeting.
The composite renal end point of the cardiovascular end point studies SUSTAIN 6 (n=3297) and LEADER (n=9340) was defined as macroalbuminuria, doubling of serum creatinine, end-stage renal failure, or death of renal cause [1,2]. The GLP-1 receptor agonists(RA) semaglutide and liraglutide showed both cardioprotective and nephroprotective potential in these two studies in patients with type 2 diabetes at high cardiovascular risk. At the EASD Virtual Meeting 2020, Prof. Johannes Mann, MD, Städtisches Klinikum München, Klinik für Nieren-, Hochdruck- und Rheumakrankheiten, spoke about a post-hoc analysis in which the mechanisms of nephroprotective effects were investigated in more detail [3].
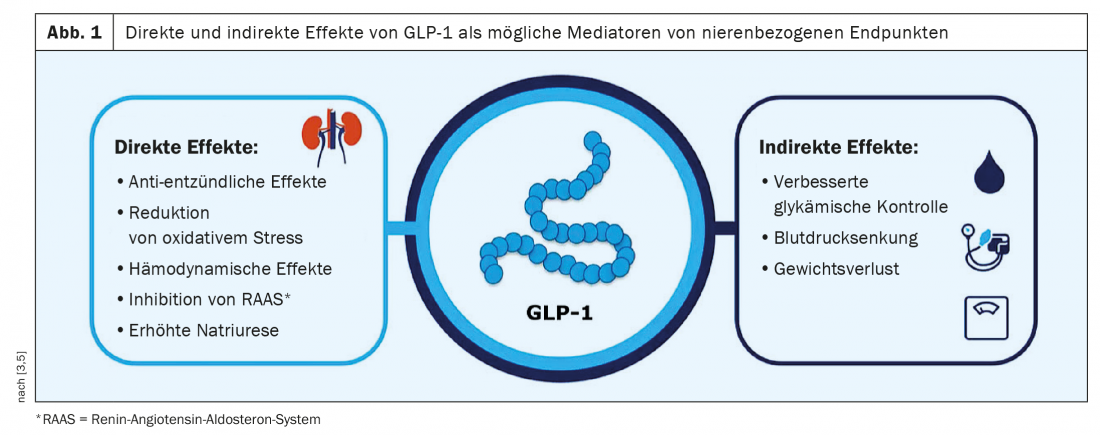
Influence of mediator variables quantified
In SUSTAIN 6 and LEADER, the rate of renal events was significantly reduced compared to placebo by 36% (semaglutide) and 22% (liraglutide), respectively. Measurable indirect effects in these two studies were improved glycemic control, blood pressure reduction, and moderate weight loss in the verum condition compared with placebo [3]. (Fig. 1). In the post-hoc analyses of the two CVOTs, Prof. Mann and his team focused on the parameters HbA1c, body weight, and systolic blood pressure as possible mediators of renal protective effects. The analyses showed that in SUSTAIN 6 over a 24-month period, 26% of the differences in the composite renal end point between verum and placebo were mediated by HbA1c. In the LEADER study, this figure was 25% over a 60-month period. These results were similar to those of a corresponding post-hoc analysis of the REWIND study, according to the speaker [3]. In contrast, the mediating effect of blood pressure was 22% in the SUSTAIN 6 study and 9% in the LEADER study [4]. Likewise, 9% were in the LEADER study with regard to body weight. In further analyses with blood pressure as a confounding variable, the value for HbA1c as a mediator variable increased to 36% and for blood pressure and body weight as confounding variables to 30% [4].
Conclusion
The mediator effects measured in the post-hoc analyses of the SUSTAIN 6 and LEADER trials were largely consistent and indicate that the renal protective effects of semaglutide and liraglutide are not exclusively attributable to HbA1c, blood pressure, and body weight but that there are other relevant, possibly direct, mechanisms. Meanwhile, the FLOW renal endpoint study was launched to investigate whether semaglutide delays the progression of chronic renal failure [6]. Renal outcomes will be examined as the primary endpoint.
Source: EASD 2020
Literature:
- Marso SP, et al: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19): 1834-1844.
- Marso SP, et al: LEADER Trial: liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311-322.
- Mann JFE: Renoprotection with Semaglutide and liraglutide – direct or indirect effects? Johannes F.E., M.D. Mann, EASD Virtual Meeting, Sept. 23, 2020
- American Society of Nephrology (ASN): abstract SA-OR082, www.asn-online.org/education/kidneyweek/2019/, last accessed 10/19/2020.
- Muskiet MHA, et al: GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetesNat Rev Nephrol 2017;13: 605-628.
- FLOW: https://clinicaltrials.gov/ct2/show/NCT038 19153
HAUSARZT PRAXIS 2020; 15(11): 47
CARDIOVASC 2020; 19(4): 35