The HER2 receptor belongs to the family of four transmembrane receptor tyrosine kinases (EGFR/HER1, HER2, HER3, and HER4) that influence cell growth, differentiation, and survival. Overexpression of HER2 occurs in approximately 20% of early breast cancers and is associated with more aggressive disease progression and poor prognosis. Only the introduction of specific agents targeting HER2 has significantly improved the prognosis of HER2-positive breast cancer patients.
The only substance approved in early-stage breast cancer is the monoclonal antibody trastuzumab (Herceptin®), according to the St. Gallen International Consensus Conference [1]. Other substances such as lapatinib (Tyverb®) and pertuzumab (Perjeta®) are currently being tested in this indication in large international trials. In the metastatic setting, these are already approved; in addition, T-DM1 (Kadcyla®) is another new therapeutic option.
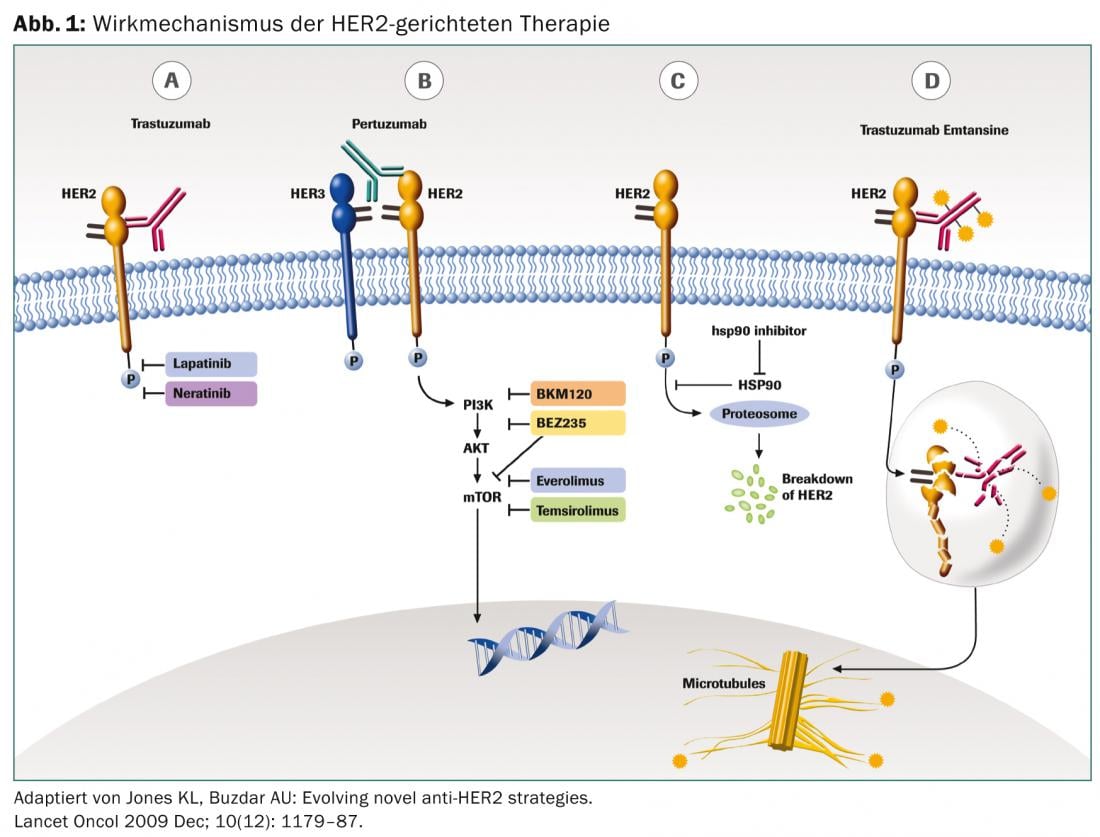
Trastuzumab
The monoclonal antibody trastuzumab targets the extracellular domain of the HER2 receptor. As a mediator of antibody-dependent cell-mediated cytotoxicity (ADCC), it prevents further proliferation. Administration is intravenous. The therapy is usually well tolerated. The first application may cause an infusion-related reaction with fever, chills and joint pain, which can be well treated with paracetamol. Cumulative cardiotoxicity is known, so echocardiography is recommended before starting therapy and at regular intervals thereafter.
Lapatinib
Lapatinib, administered perorally, reversibly inhibits the tyrosine kinase domains of EGF (ErbB1) and ErbB2 (HER2) receptors. In contrast to trastuzumab, the molecule lapatinib is also smaller, which may explain the better effect in cerebral metastases. Side effects include mainly gastrointestinal symptoms with diarrhea, loss of appetite, and nausea, as well as skin rash, fatigue, and hepatotoxicity. Cumulative cardiotoxicity has also been described with lapatinib, so appropriate monitoring is recommended.
Pertuzumab
Pertuzumab is also a monoclonal antibody and binds specifically to the extracellular dimerization domain (subdomain II) of the HER2 receptor, whereas trastuzumab binds to domain IV. Thus, pertuzumab blocks the formation of ligand-dependent heterodimerization of HER2 with other members of the HER2 family, including HER1, HER3, and HER4. Thus, pertuzumab binds to a different region of the HER2 receptor than trastuzumab, resulting in a synergistic effect. The side effect profile is similar to that of trastuzumab.
T-DM1
Trastuzumab emtansine is a conjugate of trastuzumab and the cytostatic drug mertansine, a microtubulin inhibitor. The antibody binds specifically to HER2-positive tumor cells, which is why the cytostatic causes comparatively little effect outside the tumor manifestations. This also explains the good tolerability.
The following side effects are observed: Infusion reactions with chills and fever, hematotoxicity with thrombocytopenia in particular need of control and liver enzyme elevations (ALT, AST).
Indications
Prior to any therapy, HER2 status must be determined by validated immunohistochemical examination of the tumor specimen. This can also be studied on a previously removed tumor block. Only a triple positive result is considered sufficient for anti-HER2 therapy and therefore positive. In doubtful cases (2+), HER2 gene amplification is additionally determined by fluorescence in situ hybridization (FISH), which is considered positive from a ratio >2.2 and thus qualifies for targeted therapy.
Early stage – Adjuvant treatment
At this stage, only trastuzumab has been approved so far, in combination with chemotherapy. Approval followed publication of the HERA trial in 2005, which demonstrated a significant benefit in disease-free survival and overall survival for HER2-positive breast cancer patients treated with trastuzumab over one year. Taking trastuzumab for two years does not further increase efficacy, as we have known since late 2012 [2]. Also recently shown were data from the PHARE trial, which compared six months vs. one year of trastuzumab therapy [3]. This study was negative, resp. it did not meet the primary endpoint of “non inferiority.”
Based on NOAH data, trastuzumab is also used neoadjuvantly, i.e., preoperatively in combination with chemotherapy. The 3-year relapse-free survival was significantly better at 71% compared with 56% treated without trastuzumab [4].
Adjuvant immunotherapy with trastuzumab is usually started after anthracycline-containing chemotherapy (no combination due to potential cumulative cardiotoxicity) and can be combined with non-anthracycline-containing chemotherapy, endocrine therapy, and radiotherapy.
Lapatinib is also currently being evaluated in trials. This year, a phase III study was pu-blished that compared lapatinib against trastuzumab and showed comparable progression-free survival with higher toxicity [5].
The ALTTO data, which also tested this issue and additionally a combination of trastuzumab and lapatinib, are pending, and the lapatinib arm had to be closed early. This because the lapatinib arm appears to be inferior to the trastuzumab arm.
Thus, the current standard of care in the adjuvant setting for HER2-positive breast cancer includes immunotherapy with trastuzumab for one year.
Advanced stage, metastatic
There are several therapeutic options for treatment in patients with metastatic HER2-positive breast cancer. In addition to endocrine therapy as well as cytostatic therapy, immunotherapy is of great importance here. In principle, every HER2-positive breast cancer patient should be offered appropriate targeted therapy.
Whereas until a few years ago only trastuzumab and lapatinib were available, the spectrum of therapeutic options has expanded again in recent months.
First-line therapy: In 2001, the first large phase III trial was published by Slamon, which showed significantly longer progression-free survival, better and longer response, and survival benefit for combination treatment with chemotherapy and trastuzumab [6]. A benefit was also demonstrated in combination with endocrine therapy [7].
A study by SAKK (Swiss Association for Clinical Cancer Research, www.sakk.ch) is investigating whether trastuzumab monotherapy is sufficient as the first treatment, followed by combination with chemotherapy. The results are still pending. Today, the first line of treatment is mostly trastuzumab in combination with chemotherapy or, if chemotherapy does not seem appropriate, with endocrine therapy.
Recent data from the CLEOPATRA trial have shown that docetaxel in combination with trastuzumab and pertuzumab is superior to docetaxel and trastuzumab in terms of progression-free survival as well as overall survival. However, increased toxicity must also be accepted, particularly febrile neutropenia and diarrhea, [8]. Pertuzumab is newly approved for this indication by Swissmedic and is also subject to mandatory insurance coverage after prior approval of costs.
As of second-line therapy: Despite targeted treatment, disease progression occurs under trastuzumab due to development of resistance. This development of resistance is due, among other things, to a change in the HER2 receptor and in the PI3K/Akt signaling pathway. In addition, upregulation of HER3 or IGF1R occurs [9]. However, therapy directed against HER2 should be continued according to general consensus (ESMO guidelines) [10].
One option is to continue trastuzumab with change of chemotherapy component [11, 12]. Alternatively, lapatinib (in combination with capecitabine) is available starting in second-line therapy [13]. The advantage of this therapy combination is the oral application with, however, mostly increased toxicity (especially hand-foot syndrome, gastrointestinal symptoms). In addition to lapatinib, T-DM1 is newly used from the second line onwards, which was compared against the combination of lapatinib and capecitabine and was superior in both overall survival and tolerability [14]. This has been approved in Switzerland since 2013, but is not yet subject to mandatory health insurance coverage and requires prior approval of costs.
The best therapeutic sequence in the metastatic setting is unclear. The new substances pertuzumab and T-DM1 are currently being tested in the SAKK 22/10 study. First-line therapy will be randomized to dual HER2 blockade (trastuzumab and pertuzumab) with chemotherapy vs. without chemotherapy. T-DM1 is then planned as second-line therapy.
Outlook
Subcutaneous application of trastuzumab [15] has already been tested, which may be a new and potentially attractive route of administration in the future.
Whether the new substances are also effective in the adjuvant situation is currently being investigated in large international studies.
In addition, new peroral tyrosine kinase inhibitors (TKIs) are being tested. In particular, neratinib, an irreversible EGFR-HER2 TKI, appears promising.
TAKE HOME MESSAGE
- The prognosis of HER2-positive breast carcinoma can be significantly improved by targeted immunotherapy.
- In early stage (adjuvant), trastuzumab is the current standard of care for a total of one year.
- In the palliative situation, therapy directed against HER2 should be continued even after progression. In addition to trastuzumab and lapatinib, new effective and well-tolerated therapeutic options are available with T-DM1 and pertuzumab.
A RETENIR
- Une immunothérapie ciblée peut sensiblement améliorer le pronostic du carcinome mammaire HER2 positif.
- La thérapie standard au stade précoce (adjuvant) repose actuellement sur l’administration de trastuzumab pendant un an.
- En situation palliative, la thérapie anti-HER2 doit être poursuivie, même en cas de progression. Outre le trastuzumab et le lapatinib, le T-DM1 et le pertuzumab offrent de nouvelles options de traitement efficaces et bien tolérées.
Michael Schwitter, MD
Literature:
- Goldhirsch A, et al: Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013 Sep; 24(9): 2206-23.
- Goldhirsch A, et al: 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013 Jul 17. pii: S0140-6736(13)61094-6.
- Pivot X, et al: 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013 Jul; 14(8): 741-8.
- Gianni L, et al: Neoadjuvant chemotherapy with trastuzumab followed by adjuvant tras-tuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010 Jan 30; 375(9712): 377-84.
- Goss PE, et al: Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomised, controlled, phase 3 trial. Lancet Oncol 2013 Jan; 14(1): 88-96.
- Slamon DJ, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001 Mar 15; 344(11): 783-92.
- Huober J, et al: Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer – results of the eLEcTRA trial. Breast 2012; 21: 27-33.
- Swain SM, et al: Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2013 May; 14(6): 461-71.
- Wong AL, Lee SC: Mechanisms of Resistance to Trastuzumab and Novel Therapeutic Strategies in HER2-Positive Breast Cancer. Int J Breast Cancer 2012; 2012: 415170.
- Cardoso F, et al: Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012 Oct; 23(7): vii11-9.
- von Minckwitz G, et al: Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast in-ternational group 03-05 study. J Clin Oncol 2009; 27: 1999-2006.
- Huober J, et al: Trastuzumab treatment beyond progression in advanced breast cancer: patterns of care in six Swiss breast cancer centers. Oncology 2011; 81(3-4): 160-6.
- Geyer CE, et al: Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733-2743.
- Verma S, et al: Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012 Nov 8; 367(19): 1783-91.
- Ismael G, et al: Subcutaneous versus intravenous administration of (neo)adjuvant tras-tuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 2012 Sep; 13(9): 869-78.
InFo Oncology & Hematology 2014; (2)1: 5-7.











