Results from an ongoing phase II trial of the monoclonal antibody daratumumab were presented at the ASCO Congress. This targets the protein CD38, which is highly expressed on myeloma cells. Thereafter, the drug leads to the death of the tumor cell through various processes.
In the heavily pretreated multiple myeloma population, daratumumab has been granted breakthrough therapy status by the FDA and is therefore undergoing an accelerated approval process. The present study confirms the good benefit-risk profile in this patient group. Daratumumab has also been granted orphan drug designation by the FDA and EMA.
As daratumumab is a new mode of action in myeloma therapy, the antibody raises high hopes. Data were already presented at last year’s ASCO demonstrating that daratumumab shows activity and is well tolerated as monotherapy in relapsed/refractory multiple myeloma.
MMY2002 – first part of the study
The population of the phase II trial now presented consisted of multiple myeloma patients who had undergone at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), or whose tumor was refractory to both a PI and an IMiD. This is the population for which daratumumab was granted breakthrough status. The international multicenter study is named MMY2002 and consists of two parts. The Phase II results presented at the congress should be considered preliminary, as MMY2002 has not yet been completed.
The first part was initially about dose finding. 34 patients were randomized to receive:
- Daratumumab at the dose of 8 mg/kg every four weeks (18 patients),
- Daratumumab at the dose of 16 mg/kg weekly for two months, then biweekly for four months, and finally quadweekly (16 patients).
Second part of the study: durable response and good safety
Another 90 patients were subsequently included in the second group. Data on these 106 participants in total have now been presented. The median time to diagnosis here was 4.8 years and five lines of therapy. 96% of tumors were refractory to prior therapy-95% most recently to a PI and IMiD. Failed prior therapy included pomalidomide in 63%, carfilzomib in 48%, and alkylating agents in 78%.
The primary endpoint was overall response rate, as assessed by independent reviewers. It was 29.2%. There were no differences between the clinically relevant subgroups in this regard. Three subjects achieved complete remission (sCR), ten achieved very good partial remission (VGPR), and 18 achieved partial response (PR). Median response lasted 7.4 months, with progression evident at 3.7 months. The estimated 1-year survival rate was 65%. Just over half of the responders, 45.2%, continued to receive therapy after 9.4 months of follow-up.
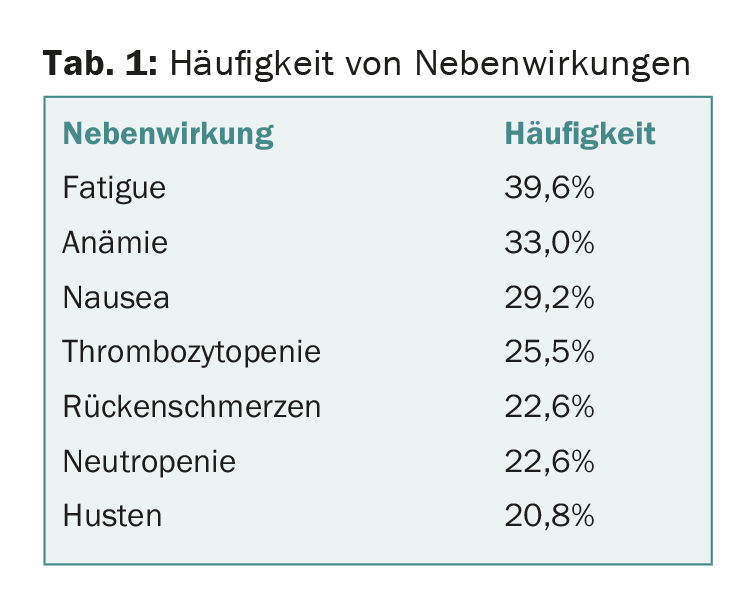
4.7% of patients discontinued treatment because of adverse events, none of which were directly associated with daratumumab. The most common adverse reactions are shown in Table 1. Infusion-related reactions occurred in 42.5% mainly during the first infusion and were predominantly mild, i.e., grade 1 and 2 (no grade 4). Grade 3 infusion reactions were found in 4.7%. None of the patients terminated therapy because of this.
Thus, with daratumumab at the dose of 16 mg/kg, a safe and active therapy is in development for heavily pretreated multiple myeloma patients. A highly welcome glimmer of hope for a population where all other available treatment options have been exhausted. Several studies are currently underway to evaluate the compound for the treatment of multiple myeloma. How the antibody works in combination with already approved drugs is also part of this research effort.
Source: ASCO Congress, May 29-June 2, 2015, Chicago.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(8): 2