Tumors of the head and neck were the seventh most common cancer worldwide in 2018. The most important risk factors include nicotine and alcohol consumption. The prognosis and multimodality treatment options for patients with head and neck tumors vary depending on epidemiologic factors, anatomic location, and tumor stage. An overview.
Tumors of the head and neck were the seventh most common cancer worldwide in 2018 (890,000 new cases) [1]. In the United States, head and neck tumors accounted for 3% of all cancers (51,540 new cases) and slightly more than 1.5% of all cancer deaths (10,030 deaths) [2]. These malignancies are most often associated with nicotine and alcohol use as major risk factors. In recent decades, there has been an overall decline in incidence, particularly due to declining nicotine use [3,4]. In contrast, an increase in oropharyngeal cancer has been demonstrated in younger individuals in North America and Northern Europe, primarily due to an increasing incidence of tumors associated with high-risk human papillomavirus (HPV) subtypes (primarily HPV type 16) [4,5].
The prognosis and multimodality treatment options for patients* with head and neck tumors vary depending on epidemiologic factors, anatomic location, and tumor stage. Head and neck tumors represent a heterogeneous group of diseases. The focus of this review article is on the treatment options for recurrent and metastatic as well as locally advanced squamous cell carcinoma originating from the mucosa (predominantly oral cavity, pharynx, and larynx). Treatment in the early stages of these tumors will not be discussed here, as there have been no relevant changes in therapy in recent years.
The treatment of patients with head and neck tumors is complex and includes not only the medical treatment of the tumor and the acute effects of surgery, chemotherapy and radiotherapy, but also the guidance and counseling in dealing with permanent impairments and the resulting psychosocial consequences. Fortunately, recent findings have led to significant improvements in treatment outcomes. Advances in surgery and radiotherapy have improved functional preservation of the corresponding organs and reduced overall morbidity and mortality. For example, newer techniques of robotic surgery for oropharyngeal carcinoma [6] and those of minimally invasive laser microsurgery for laryngeal and hypopharyngeal carcinoma [7] may relevantly increase the likelihood of functional preservation. Advances in conformal radiotherapy, such as intensity-modulated (IMRT) and image-guided ra-diotherapy, may also reduce morbidity [8]. In particular, the introduction of immune checkpoint inhibitors for the treatment of recurrent or metastatic head and neck cancer has significantly improved the prognosis of many patients*, which will be discussed first below.
Recurrent and/or metastatic head and neck tumors.
Recurrent and/or metastatic head and neck tumors are associated with a poor prognosis. Like patients with metastatic head and neck tumors, most patients* with localized primary recurrence receive palliative systemic therapy, as only selected patients* with loco-regional recurrence can be treated by surgery or re-radiotherapy [9]. Accordingly, an interdisciplinary evaluation of the disease situation, any previous treatments and the individual situation is always necessary for the individual choice of the best therapeutic strategy. For many years, the standard therapy for recurrent and/or metastatic tumors was the so-called “EXTREME regimen,” which includes platinum-based chemotherapy (cisplatin or carboplatin) in combination with fluorouracil (5-FU) and cetuximab, an antibody against the epidermal growth factor receptor (EGFR), followed by maintenance therapy with cetuximab [10]. Treatment with the EXTREME regimen showed a median overall survival of approximately ten months in the pivotal study. For patients who were not eligible for the EXTREME regimen, taxanes and methotrexate were some of the few available treatment options. With these agents, median overall survival decreases to six months [11]. These data show that new treatment options for patients with recurrent/metastatic head and neck cancer are urgently needed.
Therapies with checkpoint inhibitors have become a new standard of care in many tumor entities in recent years. Head and neck tumors are associated with immune deficits, such as altered natural killer cell function and impaired tumor-infiltrating T lymphocytes, providing the rationale for investigating immune checkpoint inhibitors in these tumors [11]. Nivolumab and pembrolizumab, both IgG4 anti-PD-1 monoclonal antibodies, were evaluated in phase III trials in patients with squamous cell carcinoma of the head and neck (oral cavity, oropharynx, larynx, or hypopharynx) after failure of platinum-based chemotherapy and compared with antibody or chemotherapy of the responsible investigator’s choice (docetaxel, cetuximab, or methotrexate). “Platinum failure” in this context was defined as disease progression within six months of platinum-containing chemotherapy used with curative (combination with radiotherapy) or palliative intent. The CheckMate 141 trial is the first phase III study to demonstrate the efficacy of nivolumab compared to chemotherapy (hazard ratio [HR] for death 0.70; p=0.01). In addition, nivolumab proved to be better tolerated (G3/4 side effects 13.1% vs. 35.1% for nivolumab and chemotherapy, respectively) and led to an improvement in quality of life [12,13]. Nivolumab was thus the first treatment option ever to significantly improve overall survival in patients with relapsed/metastatic head and neck cancer who had failed platinum-based chemotherapy [12]. In the KEYNOTE-040 trial, which was similar in design to the CheckMate-141 trial, pembrolizumab also increased overall survival compared with chemotherapy [14]. It is worth mentioning that in both studies, no evidence of PD-L1 expression was required as an inclusion criterion and the primary endpoint did not depend on PD-L1 status. None of the studies showed a significant difference in progression-free survival. Thus, similar to other solid tumors, the increase in overall survival was primarily due to the fact that immunotherapy led to long-term disease control in some patients*. For example, it was observed that while only 13% of patients* showed a radiographic response to nivolumab, the median duration of response was 9.7 months – twice as long as with chemotherapy [15]. Antibodies against PD-L1 have been studied both as monotherapy and in combination with antibodies against CTLA-4 after failure of platinum-based therapy. After initial phase II studies with durvalumab (anti-PD-L1 antibody) in patients* with high PD-L1 expression (≥25%) in the HAWK study [16] and durvalumab, durvalumab plus tremelimumab (anti-CTLA-4 antibody) or tremelimumab alone in patients* with low PD-L1 expression (<25%) in the CONDOR study [17], the phase III EAGLE study was initiated [18]. In this study, patients with recurrent/metastatic head and neck cancer after failure of platinum-based therapy were randomized between durvalumab plus tremelimumab, durvalumab monotherapy, or standard chemotherapy of the study physician’s choice. The study showed no survival benefit with durvalumab therapy (HR 0.88; p=0.20) or durvalumab plus tremelimumab treatment (HR 1.04; p=0.76) compared to chemotherapy. Taking into account the limited power of inter-study comparisons, it is noteworthy that median overall survival in the durvalumab arm was similar to that seen with nivolumab in the CheckMate-141 trial (7.6 and 7.5 months, respectively), but median overall survival in the control arm was numerically longer in the EAGLE trial compared with the CheckMate-141 trial (8.3 months and 5.1 months, respectively). Exploratory analysis of the EAGLE study suggests that this higher than expected overall survival in the control group may be due to unbalanced characteristics at study inclusion (higher percentage of patients with ECOG PS 0 and distant metastases without local/regional recurrence in the control group), increased use of paclitaxel in the control group, and subsequent treatment with anti-PD-1 antibodies. [18]. To what extent the difference between PD-1 and PD-L1 antibodies has clinical relevance is currently unclear.
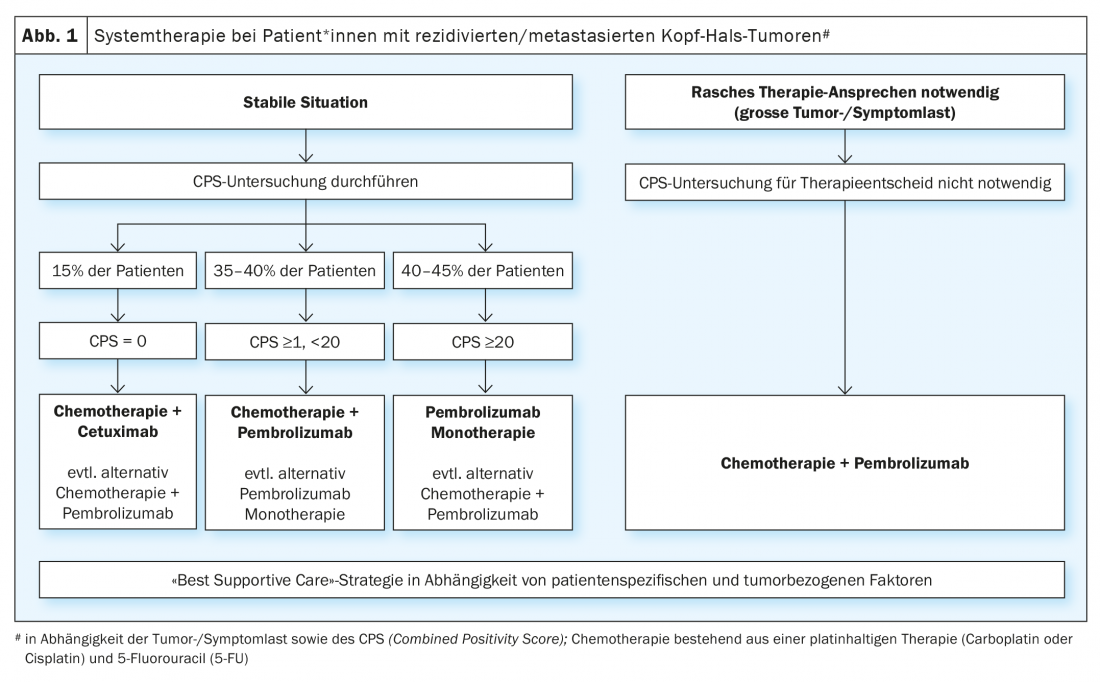
The demonstrated benefit of immune checkpoint inhibitor therapy in patients with recurrent/metastatic head and neck cancer after failure of platinum-based therapy led to the evaluation of these agents in first-line palliative therapy. The randomized phase III KEYNOTE-048 trial compared monotherapy with pembrolizumab and combination treatment with platinum/5-FU and pembrolizumab with the EXTREME regimen (platinum/5-FU/cetuximab) [19]. Statistical analyses for overall survival were performed in the total population and in defined subgroups depending on PD-L1 expression. PD-L1 expression was graded according to the so-called “combined positivity score ” (CPS ) (CPS ≥1 and ≥20). The CPS is defined as the number of PD-L1 positive cells (tumor cells, lymphocytes, macrophages) divided by the total number of tumor cells and multiplied by a factor of 100. Monotherapy with pembrolizumab significantly improved overall survival in patients* with CPS ≥1 and ≥20. Although the response rate was lower than with chemotherapy (19-21% vs. 36%), the median duration of response was quintupled with pembrolizumab monotherapy (20.9 vs. 4.5 months). Chemotherapy plus pembrolizumab significantly improved overall survival in all three populations. There was no significant difference in response rate and progression-free time between the combination treatment of chemotherapy plus pembrolizumab and the EXTREME regimen. As expected, pembrolizumab monotherapy was associated with less toxicity, whereas platinum/5-FU plus pembrolizumab had a similar rate of adverse events as the EXTREME regimen. The phase III KESTREL trial randomized patients* in a 2:1:1 ratio to durvalumab alone, durvalumab plus tremelimumab, or the EXTREME regimen (NCT02551159). The primary endpoint is overall survival with durvalumab monotherapy compared to EXTREME in patients* with high PD-L1 expression (defined as expression of > 50% in tumor cells or >25% in tumor infiltrating lymphocytes). The secondary endpoint is overall survival with durvalumab plus tremelimumab compared to EXTREME for all patients. No data has been published yet. However, it was announced via press release that the study did not meet its endpoints.
In summary, for first-line therapy, the KEYNOTE-048 study represents the first positive study since the publication of the EXTREME study in 2009 and has changed the standard of care. However, there are still some open questions that affect the daily practice. One is whether patients* with a CPS ≥20, who accounted for 44% of patients* with proven PD-L1 expression in the KEYNOTE-048 trial, were the reason for the benefit of monotherapy with pembrolizumab in the CPS ≥1 group. The question of whether monotherapy with pembrolizumab is sufficient for patients* with CPS 1-19 cannot be answered unequivocally. A subgroup analysis of the KEYNOTE-048 trial showed that pembrolizumab still had a benefit compared with EXTREME in CPS 1-19 (HR 0.86), although smaller than in patients* with CPS ≥20 (HR 0.58) [20]. In clinical practice, the decision for monotherapy with pembrolizumab versus combined therapy with platinum/5-FU plus pembrolizumab for patients with CPS 1-19 primarily depends on the tumor and symptom burden. In addition, comorbidities and the general condition of the affected person should also be taken into account. In patients* with CPS 1-19 as well as high tumor burden and/or significant tumor-related symptoms, combined chemo-immunotherapy is often preferred to maximize potential response. Another question is about the treatment sequence of PD-L1-negative patients, who make up about 15% of those affected. A subgroup analysis for PD-L1-negative patients* in the KEYNOTE 048 trial showed an advantage for the EXTREME regimen compared with combination treatment with pembrolizumab (HR 1.22) [20]. However, given the small number of patients in this cohort and the fact that this was an unplanned subgroup analysis, it cannot be concluded that PD-L1-negative patients* should not be treated with the combination of platinum/5-FU and pembrolizumab. In summary, monotherapy with pembrolizumab in patients with high PD-L1 expression (CPS ≥20) and combination therapy of platinum/5-FU and pembrolizumab represent a new standard of care regardless of PD-L1 expression, but especially in PD-L1-positive tumors (Fig. 1). For patients who show tumor progression under or after this first-line therapy, the above-mentioned chemotherapies (docetaxel, methotrexate) or treatment with cetuximab are available treatment options. Numerous studies are currently investigating new immunotherapeutic approaches. For example, the INTERLINK-1 trial is open for recruitment in several centers in Switzerland. This randomized trial compares cetuximab with the combination of cetuximab and the immune checkpoint inhibitor monalizumab directed against NKG2A (NCT04590963).
In addition to PD-L1 expression, HPV status could also serve as a clinical biomarker for predicting response to immune checkpoint inhibitor therapy. HPV infection leads to the production of virus-related proteins that can trigger a de novo T-cell response and greater infiltration of the tumor with CD8+ T cells [21]. In the phase II KEYNOTE-055 trial, the response rate to pembrolizumab was 22% in patients* with p16-positive tumors and 16% in p16-negative tumors [22]. A meta-analysis also showed that HPV status appears to correlate with response rate to anti-PD-1 treatment independent of PD-L1 expression and tumor mutation burden [23]. Another meta-analysis concluded that patients with HPV-positive tumors had significantly better outcomes with immune checkpoint inhibitor therapy [24]. Whether these results have any relevance in clinical practice cannot be definitively assessed at present. The current guidelines on immunotherapy for head and neck tumors do not yet recommend using HPV status for treatment decisions [25].
Locally advanced head and neck tumors
More than 60% of patients* with squamous cell carcinoma of the head and neck have stage III or IV disease, which is characterized by large tumors with marked local invasion, evidence of metastasis to regional lymph nodes, or both. Locally advanced disease carries a high risk of local recurrence (15% to 40%) and distant metastasis [26]. Multimodal approaches have improved cure rates while striving to preserve function and quality of life [27]. Therapeutic decisions at these stages require a complex balancing of morbidity, potential side effects, and preservation of function. They also depend strongly on the size and anatomical location of the primary tumor, the stage of the disease, the age of the affected person, their preferences as well as their general condition and concomitant diseases. These therapeutic decisions should therefore always be made on an interdisciplinary basis within the framework of a tumor board.
In locally advanced, resectable tumors, adjuvant therapy consisting of radiotherapy or combined radio-chemotherapy (RCT) follows resection with curative intent. This depends on the risk factors and comorbidities of the affected persons. Microscopic evidence of tumor cells on the resectate (R1) and evidence of extracapsular growth in the lymph nodes are considered “high risk” risk factors. When these are present, adjuvant combined RCT is recommended. Cisplatin has been established as a chemotherapeutic agent in this setting [28,29].
When surgical resection is not feasible technically or because of comorbidities, or would result in potentially poor long-term functional outcomes, definitive RCT is the established curative standard. A large meta-analysis (Meta-analysis of Chemotherapy in Head and Neck Cancer [MACH-NC]) included nearly 20 000 patients with locally advanced head and neck cancer. Combined RCT versus radiotherapy alone was shown to reduce 5-year mortality by 6.5 percentage points (HR 0.83; p<0.001). The addition of induction or adjuvant chemotherapy did not significantly improve overall survival compared with RCT alone [30]. High-dose cisplatin (100 mg/m2 body surface area, administered intravenously every 21 days for three cycles) given concurrently with radiotherapy as part of definitive RCT is the current standard of care. However, due to the significant short- and long-term toxicities associated with cisplatin, its use is reserved primarily for younger patients* who do not have serious comorbidities [30–32]. This resulted in several modifications and variations of the originally used regimen of cisplatin (once every three weeks). Systematic reviews compared data between cisplatin once weekly and cisplatin every three weeks (inclusion criterion: cumulative cisplatin dosage of ≥180 mg/m2). This showed similar results and lower rates of serious adverse events, particularly nephrotoxicity and ototoxicity [33]. We were also able to confirm this in our own analysis, although the cumulative dose of cisplatin was lower in patients* treated at weekly intervals [34]. Therefore, weekly cisplatin therapy can be used as an acceptable alternative because the dosage is more controllable and nephrotoxicity and ototoxicity are generally less severe. In addition, in patients* who are not eligible for cisplatin (e.g., patients with chronic kidney disease, chronic hearing loss, advanced age, or borderline ECOG performance status), carboplatin is often used in combination with a taxane, although there is no direct comparison in randomized controlled trials. Cetuximab administered concurrently with radiotherapy was approved as standard therapy back in 2006 after data showed that this treatment resulted in improved loco-regional control and overall survival compared with radiotherapy alone [35]. However, recent randomized trials suggest that combined radiotherapy with cetuximab in patients* with HPV-positive oropharyngeal carcinoma has worse outcomes, including lower survival, when compared directly with high-dose cisplatin in combination with radiotherapy [36,37]. Thus, combined RCT with cisplatin remains the preferred standard therapy.
Another promising approach to improve prognosis is to combine cisplatin-based RCT with xevinapant. Xevinapant is an antagonist of apoptosis inhibitors. In a randomized phase II trial, the addition of xevinapant to RCT significantly improved loco-regional tumor control rate as a primary endpoint at the 18-month time point by 21% compared to placebo in combination with RCT (54% vs. 33%; p=0.026) [38]. In addition, after two years of follow-up, a significant benefit in terms of progression-free survival was observed compared to the control arm (HR 0.37; p=0.0069). After a follow-up period of three years, Xevinapant plus RCT showed a statistically significant 51% reduction in the risk of death compared with placebo plus RCT (HR 0.49; p=0.0261). To confirm these results, the randomized Phase III TrilynX trial (NCT04459715) is currently ongoing.
Based on the encouraging data in relapsed/metastatic head and neck tumors, numerous studies are currently evaluating the use of immune checkpoint inhibitors also in earlier stages, especially in locally advanced tumors in combination with RCT. For example, the multinational phase III JAVELIN Head and Neck 100 trial [39] already evaluated the efficacy of avelumab, a PD-L1 inhibitor, in combination with RCT with cisplatin compared with placebo in combination with RCT. The trial was stopped early after an interim analysis because it showed no improvement in progression-free time [40]. An ongoing study is investigating the value of maintenance therapy with atezolizumab after completion of the RCT (IMvoke010; NCT03452137). Additional studies are testing combined RCT with immune checkpoint inhibitors followed by maintenance therapy. The largest of these trials is KEYNOTE-412 (pembrolizumab vs. placebo; NCT03040999).
Another interesting approach that has been investigated in various tumors and has led to promising results is the neoadjuvant use of immune checkpoint inhibitors [41,42]. Considering the naive treatment situation and the lack of treatment-resistant cells compared to the relapsed/metastatic situation, neoadjuvant immunotherapy may be able to provide a more potent and durable therapeutic effect. For example, neoadjuvant anti-PD-1 treatment in a mouse model of head and neck tumors resulted in a conversion of functional immunodominance and induced robust immune responses directed against the tumor [43]. Schoenfeld et al. investigated nivolumab (N) neoadjuvantly in a phase II study and the combination of nivolumab plus ipilimumab (N+I) in a second cohort of 29 untreated patients with oral cavity carcinoma [44]. Nivolumab (3 mg/kg) was administered at weeks 1 and 3, whereas ipilimumab (1 mg/kg) was administered at week 1 only. Although a total of 21 patients* experienced adverse events, including grade 3/4 in 2 (N) and 5 (N+I) patients*, there were no delays in surgery. In addition, there was evidence of response to these therapies in both cohorts. Notably, four patients* (N, n=1; N+I, n=3) had a complete or near-complete (<10% vital tumor cells) response. These results support the clinical tolerability and efficacy of neoadjuvant immunotherapy. Other interesting data on the neoadjuvant use of immune checkpoint inhibitors were provided by the CAIO study [45], the IMCISION study [46], the CheckMate 358 study [47] as well as other studies [48–50], all of which demonstrated the feasibility and efficacy of this therapeutic strategy. Accordingly, larger studies have been initiated based on this (e.g. randomized phase III study KEYNOTE-689, NCT03765918). In addition, it is important to explore possible predictors of response to enable selection of appropriate patients. Here, a detailed analysis of the tumor samples as well as of possible changes in the tumor microenvironment and of tumor-infiltrating immune cells is of importance.
Summary/Conclusion
The introduction of immunotherapy into the treatment of recurrent and/or metastatic head and neck cancer has fundamentally changed the treatment of these conditions and significantly improved their prognosis. Treatment analogous to the KEYNOTE-048 trial (platinum-containing chemotherapy + 5-FU + pembrolizumab or pembolizumab mono according to CPS and tumor/symptom burden) has become established as the new palliative first-line therapy. Based on encouraging results in advanced tumor stages, the question of the benefit of immunotherapy also arises for earlier tumor stages, in combination or sequence to radiotherapy and chemotherapy. It is very likely that the sequence of different treatment modalities significantly affects outcomes. Especially considering that the anti-tumor T-cell response has different phases that can be targeted by different immunotherapies, and that both radio- and chemotherapy can modulate the immune system while having cytotoxic effects against T cells [51]. Other novel combination approaches are being explored in clinical trials combining immunotherapeutics with vaccines against HPV, patient-specific tumor vaccines, T-cell targeted therapies, oncolytic viruses and other immunomodulators.
Take-Home Messages
- The introduction of immunotherapy with immune checkpoint inhibitors in the treatment of recurrent/metastatic head and neck cancer has significantly improved the prognosis of these patients.
- Monotherapy with the anti-PD-1 antibody pembrolizumab in patients* with high PD-L1 expression (CPS ≥20) and combination therapy of platinum/5-FU and pembrolizumab (independent of PD-L1 expression, but especially with a CPS ≥1) represent the new standard of care in relapsed/metastatic head and neck tumors based on the KEYNOTE-048 trial.
- For locally advanced, unresectable tumors, definitive combined radio-chemotherapy (RCT) with cisplatin remains the standard therapy. Drug alternatives to cisplatin in combination with radiotherapy are carboplatin combined with a taxane and cetuximab. Xevinapant (antagonist of apoptosis inhibitors) complementary to RCT seems promising (randomized phase III trial currently ongoing).
- The use of immunotherapy in earlier tumor stages in combination with RCT, as maintenance therapy or in the neoadjuvant setting, is the subject of ongoing studies.
Conflicts of interest
ED:
No conflicts of interest.
SR: Fees for consulting services (payment to University Hospital Basel) from Astra-Zeneca, BMS, Boehringer-Ingelheim, Eisai, Eli Lilly, Merck Serono, MSD, Novartis, Pfizer, Roche, Takeda. Research funding from AbbVie, Astra-Zeneca, BMS, Boehringer-Ingelheim, Merck. Support for congress participation from Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, MSD, Roche, Takeda. Member of the Federal Drug Commission of the Federal Office of Public Health.
Literature:
- Bray F, et al: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424.
- Siegel RL, et al: An assessment of progress in cancer control. CA Cancer J Clin 2018; 68: 329-339.
- Mourad M, et al: Epidemiological Trends of Head and Neck Cancer in the United States: A SEER Population Study. J Oral Maxillofac Surg 2017; 75: 2562-2572.
- Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden of Disease Cancer Collaboration. JAMA Oncol. 2017; 3: 524-548.
- Gillison ML, et al: Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015; 33: 3235-3242.
- Moore EJ, Janus J, Kasperbauer J: Transoral robotic surgery of the oropharynx: Clinical and anatomic considerations. Clin. Anat. 2012; 25: 135-141.
- Weiss BG, et al: Transoral laser microsurgery for treatment for hypopharyngeal cancer in 211 patients. Head Neck 2017; 39: 1631-1638.
- Gupta T, et al: Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS One. 2018; 13. DOI:10.1371/journal.pone.0200137.
- Vermorken JB, Specenier P: Optimal treatment for recurrent/metastatic head and neck cancer. Annals of Oncology. Oxford University Press, 2010. DOI:10.1093/annonc/mdq453.
- Vermorken JB, et al: Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359: 1116-1127.
- Saleh K, et al: New developments in the management of head and neck cancer – Impact of pembrolizumab. Ther. Clin. Risk Manag. 2018; 14: 295-303.
- Ferris RL, et al: Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016; 375: 1856-1867.
- Harrington KJ, et al: Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017; 18: 1104-1115.
- Cohen EEW, et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019; 393: 156-167.
- Ferris RL, et al: Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018; 81: 45-51.
- Zandberg DP, et al: Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumor cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019; 107: 142-152.
- Siu LL, et al: Safety and Efficacy of Durvalumab with or Without Tremelimumab in Patients with PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol 2019; 5: 195-203.
- Ferris RL, et al: Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020; 31: 942-950.
- Burtness B, et al: Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019; 394: 1915-1928.
- Burtness B, et al: Abstract LB-258: Efficacy of first-line (1L) pembrolizumab by PD-L1 combined positive score <1, 1-19, and ≥20 in recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): KEYNOTE-048 subgroup analysis. Cancer Research. American Association for Cancer Research (AACR), 2020.
- Matlung SE, et al: Differences in T-cell infiltrates and survival between HPV+ and HPV- oropharyngeal squamous cell carcinoma. Futur. Sci. OA. 2016; 2. DOI:10.4155/fso.15.88.
- Bauml J, et al: Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol 2017; 35: 1542-9.
- Wang J, et al: HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep 2019; 9. DOI:10.1038/s41598-019-49771-0.
- Xu Y, et al: Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis. Front Immunol 2021; 12. DOI:10.3389/fimmu.2021.645170.
- Cohen EEW, et al: The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 2019; 7. DOI:10.1186/s40425-019-0662-5.
- Braakhuis BJM, Brakenhoff RH, René Leemans C: Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann Oncol 2012; 23. DOI:10.1093/annonc/mds299.
- Brana I, Siu LL: Locally advanced head and neck squamous cell cancer: treatment choice based on risk factors and optimizing drug prescription. Ann Oncol 2012; 23. DOI:10.1093/annonc/mds322.
- Cooper JS, et al: Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350: 1937-1944.
- Bernier J, et al: Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350: 1945-1952.
- Pignon JP, et al: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4-14.
- Adelstein D, et al: NCCN guidelines® insights head and neck cancers, version 2.2017 featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw 2017; 15: 761-770.
- Adelstein DJ, et al: An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92-98.
- Mohamed A, et al: Concurrent chemoradiotherapy with weekly versus triweekly cisplatin in locally advanced squamous cell carcinoma of the head and neck: Comparative analysis. Head Neck. 2019; 41: 1490-1498.
- Helfenstein S, et al: 3-weekly or weekly cisplatin concurrently with radiotherapy for patients with squamous cell carcinoma of the head and neck – A multicentre, retrospective analysis. Radiat Oncol 2019; 14. DOI:10.1186/s13014-019-1235-y.
- Bonner JA, et al: Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11: 21-28.
- Mehanna H, et al: Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019; 393: 51-60.
- Gillison ML, et al: Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019; 393: 40-50.
- Bourhis J, et al: TrilynX: A phase 3 trial of xevinapant and concurrent chemoradiation for locally advanced head and neck cancer. J Clin Oncol 2021; 39: TPS6091-TPS6091.
- Yu Y, Lee NY: JAVELIN Head and Neck 100: A phase III trial of avelumab and chemoradiation for locally advanced head and neck cancer. Futur Oncol 2019; 15: 687-694.
- Cohen EE, et al: Primary results of the phase III JAVELIN head & neck 100 trial: avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). ESMO Congress 2020: Annals of Oncology (2020) 31 (suppl_4): S599-S628.
- Topalian SL, Taube JM, Pardoll DM: Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020; 367. DOI:10.1126/science.aax0182.
- Rothschild SI, et al: SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J Clin Oncol 2021; 39: 2872-2880.
- Friedman J, et al: Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res 2020; 26: 679-689.
- Schoenfeld JD, et al: Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol 2020; 6: 1563-1570.
- Ferrarotto R, et al: Impact of neoadjuvant durvalumab with or without tremelimumab on CD8+ tumor lymphocyte density, safety, and efficacy in patients with oropharyngeal cancer: CIAO trial results. Clin Cancer Res 2020; 26: 3211-3219.
- Vos JL, et al: Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun 2021; 12. DOI:10.1038/s41467-021-26472-9.
- Ferris RL, et al: Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Immunother Cancer 2021; 9. DOI:10.1136/jitc-2021-002568.
- Merlino DJ, et al: Discordant Responses Between Primary Head and Neck Tumors and Nodal Metastases Treated With Neoadjuvant Nivolumab: Correlation of Radiographic and Pathologic Treatment Effect. Front Oncol 2020; 10. DOI:10.3389/fonc.2020.566315.
- Xiong Y, et al: Immunological effects of nivolumab immunotherapy in patients with oral cavity squamous cell carcinoma. BMC Cancer 2020; 20. DOI: 10.1186/s12885-020-06726-3.
- Uppaluri R, et al: Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial. Clin Cancer Res 2020; 26: 5140-52.
- Yan Y, et al: Combining Immune Checkpoint Inhibitors With Conventional Cancer Therapy. Front. Immunol. 2018; 9. DOI:10.3389/fimmu.2018.01739.
InFo ONCOLOGY & HEMATOLOGY 2022; 10(1): 5-11.