No ESC Congress without new studies on antithrombotic therapy after percutaneous coronary intervention. Recently, monotherapy with a P2Y12 blocker has increasingly emerged as an attractive alternative to dual antiplatelet therapy (DAPT) with a P2Y12 blocker plus ASA due to the lower risk of bleeding. The STOPADAPT-3 study [2] is concerned with the question of whether it is possible to manage without ASA altogether.
Patients with acute coronary syndrome (ACS) have been reported to have a higher risk of ischemic events [3]. Therefore, the current guidelines recommend newer and more effective P2Y12 receptor inhibitors such as ticagrelor and prasugrel in patients with ACS, while clopidogrel is still recommended in patients with stable coronary artery disease (CAD) [4,5]. In ACS patients, the current guidelines also recommend the use of anticoagulants prior to percutaneous coronary intervention (PCI), the administration of antiplatelet agents and prolonged dual antiplatelet therapy (DAPT) if there is no high risk of bleeding [4,5]. In addition, the transfemoral approach is often chosen for PCI in ACS, which has been reported to be associated with a higher risk of bleeding [6]. Therefore, patients with ACS may also have a higher risk of bleeding than patients with stable CHD. Nevertheless, the clinical presentation of ACS was not included as a criterion in the Academic Research Consortium (ARC)-HBR, partly because there were no previous studies comparing the risk of bleeding after PCI between ACS and stable CHD patients [7].
A meta-analysis of randomized trials with ACS patients reported that the new generation of drug-eluting stents (DES) reduces the long-term risk of stent thrombosis, myocardial infarction and cardiac death compared to bare-metal stents [8]. The balance between ischemia and bleeding risk after PCI in ACS patients may have changed significantly in the era of new-generation DES.
Design and results of STOPDAPT-3
STOPDAPT-3 was designed to investigate whether early cessation of DAPT after DES implantation in ACS with HBR can reduce major bleeding without increasing the risk of cardiovascular complications.
The 13,258 patients included 5521 patients (42%) with ACS (ACS group) and 7737 patients (58%) with stable CHD (stable CHD group). In the ACS group, there were 4081 patients (74%) with STEMI and 1440 patients (26%) with non-ST-elevation ACS (NSTEACS), including 1235 patients (22%) with NSTEMI and 205 patients (3.7%) with UA. Patients were categorized into four combination groups based on clinical presentation (ACS, stable CHD) and the presence of ARC-HBR (ACS/HBR: 2502 patients; ACS/No-HBR: 3019 patients; stable CHD/HBR: 3905 patients; stable CHD/No-HBR: 3832 patients).
Basic characteristics: ACS vs. stable CHD
In the present study population, 48% of patients had ARC-HBR. The patients in the ACS group were younger, often male and smokers, had a lower body mass index and suffered more frequently from heart failure and severe frailty than the patients in the stable CHD group.
In terms of procedural characteristics, the total number of stents was greater, the total length of stents was longer, and the minimum stent size was smaller in the stable CHD group than in the ACS group. Transfemoral access was chosen more frequently in the ACS group than in the stable CHD group. In addition, patients in the ACS group received statins, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, oral anticoagulants and proton pump inhibitors/H2-blockers more frequently than patients in the stable CHD group. The prevalence of high-intensity statin therapy was very low in both groups.
Long-term clinical results: ACS vs. stable CHD
The median follow-up time for survivors was 6.0 years, and complete 1-, 3-, and 5-year clinical follow-up data were obtained for 96.9%, 93.4%, and 78.6% of patients, respectively. The cumulative incidence of permanent discontinuation of DAPT was significantly higher in the ACS group than in the stable CHD group, suggesting that the duration of DAPT was significantly shorter in the ACS group than in the stable CHD group.
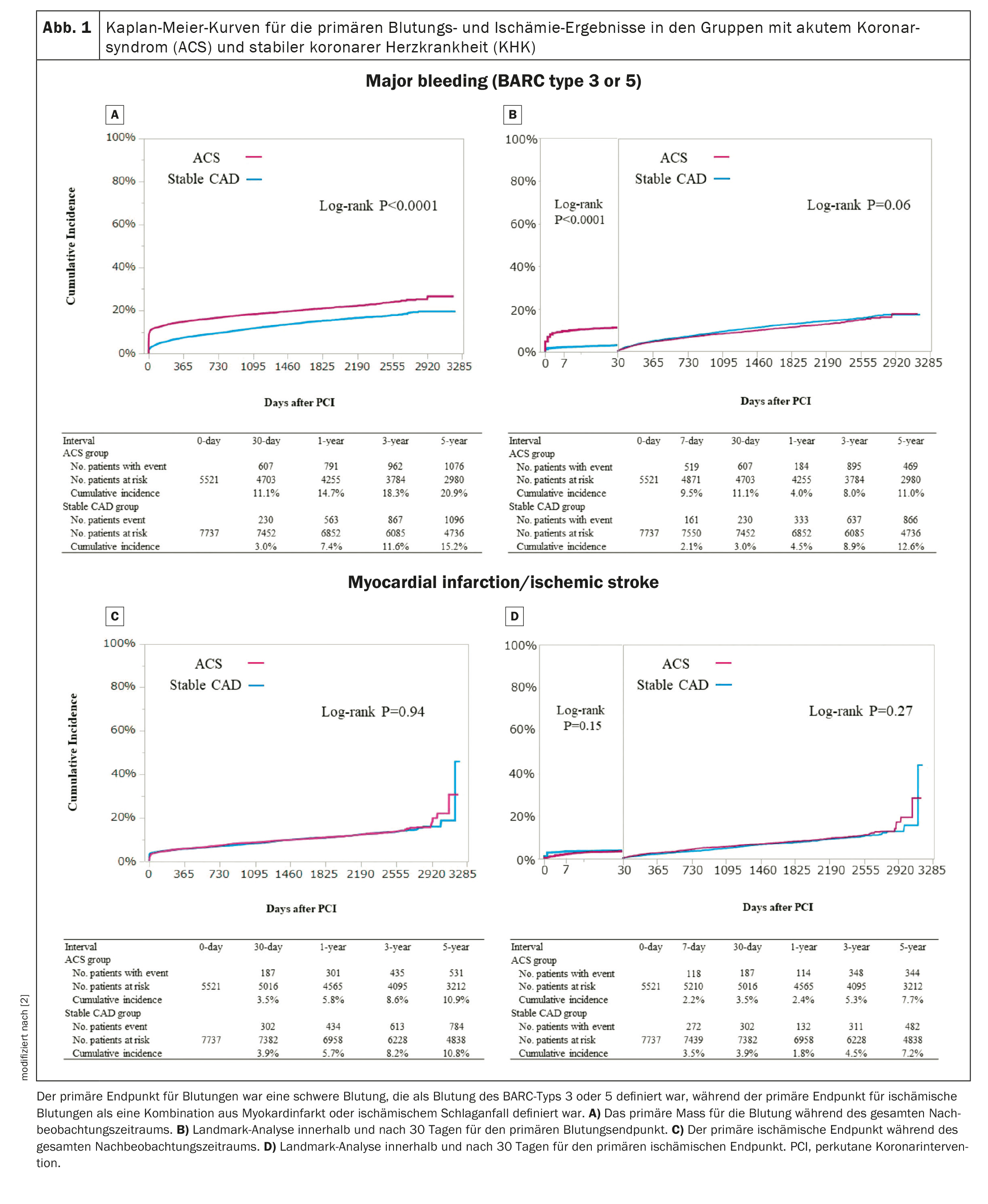
The cumulative 5-year incidence of the primary bleeding outcome (BARC type 3 or 5 bleeds) was significantly higher in the ACS group than in the stable CHD group (Fig. 1A) [2]. In the 30-day landmark analysis, the cumulative incidence of the primary bleeding outcome within 30 days was also significantly higher in the ACS group than in the stable CHD group (Fig. 1B) [2]. After adjusting for confounders, the increased risk of ACS compared to stable CHD for the primary bleeding measure remained significant throughout the follow-up period and within 30 days, while it was no longer significant after 30 days. In terms of bleeding types, the cumulative incidence of access site bleeding, gastrointestinal bleeding and other bleeding was significantly higher in the ACS group than in the stable CHD group, while the cumulative incidence of intracranial bleeding did not differ between the two groups. The cumulative incidence of the primary outcome measure of bleeding did not differ between STEMI and NSTEACS.
The cumulative 5-year incidence of the primary ischemic endpoint did not differ significantly between the two groups during the entire follow-up period or within 30 days (Fig. 1C, 1D) [2]. After adjusting for construct, the increased risk of ACS compared to stable CHD for the primary ischemic outcome measure remained nonsignificant. The risk of ACS compared to stable CHD for the primary ischemic endpoint was significantly lower within 30 days, but higher after 30 days. The increased risk of ACS compared to stable CHD was significant for all causes of death, cardiac death, cardiovascular death, non-cardiovascular death, ischemic stroke, definite stent thrombosis and target vessel revascularization, but not for myocardial infarction according to the ARTS definition. The cumulative incidence of primary ischemic outcome did not differ between STEMI and NSTEACS.
Initial characteristics depending on clinical picture and HBR
Baseline characteristics and drug treatment differed significantly between the four categories according to clinical history (ACS and stable CHD) and the presence of ARC-HBR. The patients in the ACS/HBR group were older, had a lower body mass index and suffered more frequently from heart failure and severe frailty.
Long-term clinical results depending on clinical presentation and HBR
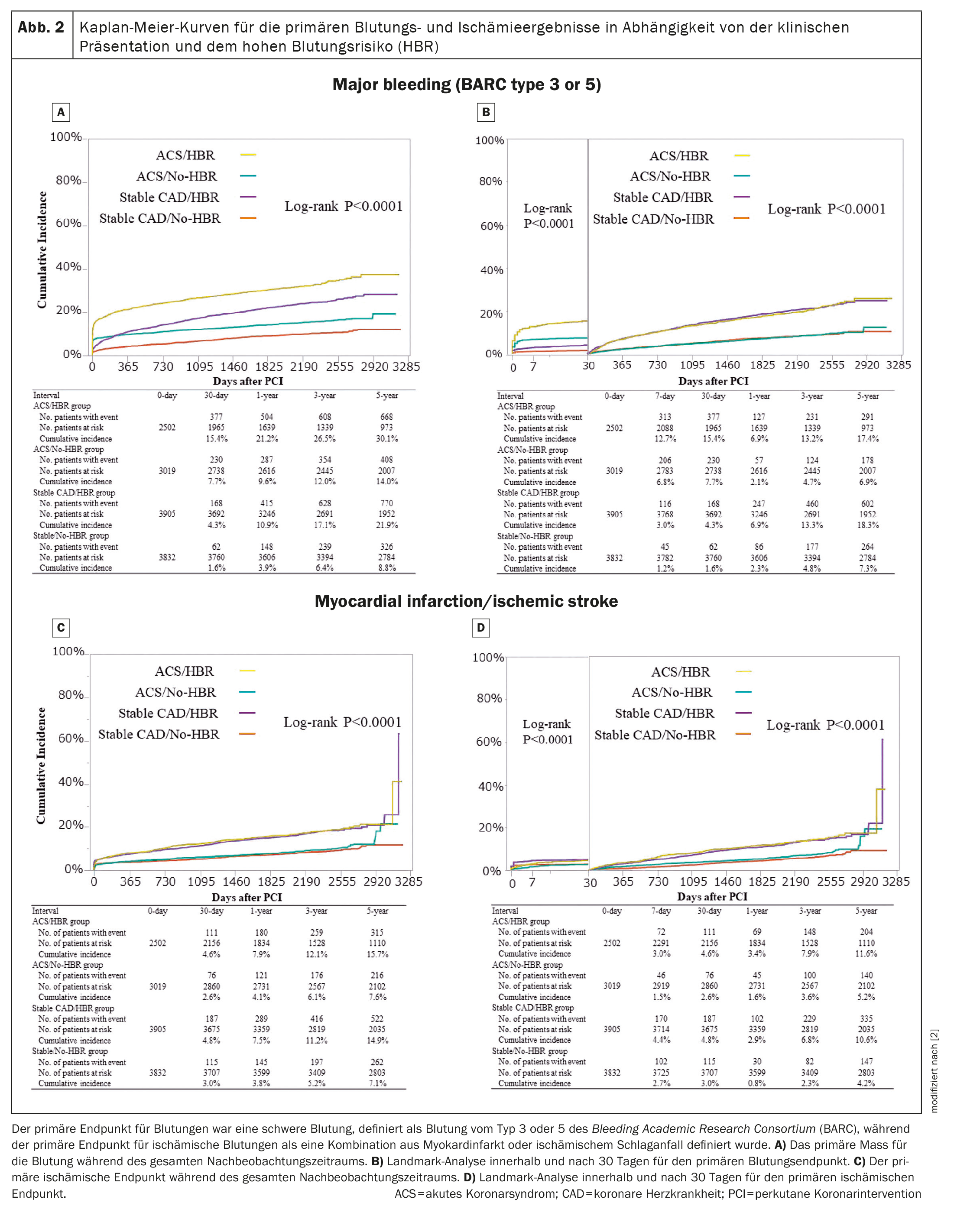
In all four groups, the cumulative incidence of permanent discontinuation of DAPT was highest in the ACS/HBR group. The cumulative 5-year incidence of the primary bleeding outcome decreased in the order of ACS/HBR, stable CHD/HBR and ACS/No-HBR, followed by the stable CHD/No-HBR group (Fig. 2A) [2]. Within 30 days, the cumulative incidence of the primary bleeding outcome was higher in the two ACS groups with and without HBR than in the two groups with stable CHD with and without HBR (Fig. 2B) [2]. The adjusted risk for the primary bleeding outcome was much higher in the ACS/HBR group and moderately higher in the ACS/No-HBR and stable CHD/HBR groups than in the stable CHD/No-HBR group (HR 3.05 [95% CI 2.64-3.54; p<0.0001], HR 1.69 [95% CI 1.45-1.98; p<0.0001] and HR 1.89 [95% CI 1.66-2.15; p<0.0001]).
Within 30 days, the adjusted risk for the primary bleeding outcome was significantly higher in the two ACS groups with and without HBR and moderately higher in the stable CHD/HBR group than in the stable CHD/no-HBR group. At 30 days, the adjusted risk for the primary bleeding outcome was significantly higher in the two HBR groups with and without ACS than in the stable CHD/non-HBR group.
The cumulative 5-year incidence of the primary ischemic outcome was significantly higher in the two HBR groups than in the two non-HBR groups throughout the follow-up period. The cumulative 5-year incidence of the primary ischemic outcome was significantly higher in the two HBR groups than in the two no-HBR groups during the entire follow-up period (Fig. 2C) [2] and within and after 30 days (Fig. 2D) [2]. The adjusted risk for the primary ischemic outcome was slightly higher in the two HBR groups than in the stable CHD/no-HBR group, while it did not differ significantly between the ACS/no-HBR and stable CHD/no-HBR groups.
Primary bleeding result after 30 days depending on access site
The cumulative 30-day incidence of the primary bleeding outcome (BARC type 3 or 5 bleeding) was significantly higher in patients with femoral access than in patients with radial access.
Aspirin remains the “cornerstone” of treatment
Study author Dr. Masahiro Natsuaki from Saga University in Japan, summarized the results of the STOPDAPT-3 trial as follows: “The aspirin-free strategy failed to reduce major bleeding within one month after PCI compared to the DAPT strategy, but was non-inferior in terms of the co-primary cardiovascular endpoint with a relative margin of 50%. Aspirin, used as a component of DAPT for a limited period of one month after PCI, may have exerted a protective effect on vulnerable coronary lesions, particularly in patients with ACS, without a large increase in major bleeding. DAPT should remain the standard strategy for PCI in the era of new generation drug-eluting stents.” [1]
Discussing the STOPDAPT-3 results after the hot-line presentation, Marco Valgimigli, deputy head of interventional cardiology at the Cardiocentro Ticino Institute in Lugano, Switzerland, said the STOPDAPT-3 data show no benefit for major bleeding and a signal of potential harm in terms of subacute stent thrombosis if aspirin therapy is omitted after PCI. “The absolute event rates were extremely low at 0.2% versus 0.6%, but undoubtedly higher in the group without aspirin,” Valgimigli concluded. “The implications for clinical practice are clear. Aspirin remains a cornerstone in the periprocedural and acute phase of PCI in patients without an indication for oral anticoagulation.”
The guidelines recommend a DAPT of six months for patients with ACS and HBR, and 12 months without HBR. For non-ACS patients, the guideline recommends a DAPT of 1-3 months.
Congress: ESC 2023
Literature:
- Natsuaki M: STOPDAPT-3: An Aspirin-Free antithrombotic strategy for percutaneous coronary intervention. Hot Line Session 3, ESC Congress 2023, Amsterdam, 26.08.2023.
- Natsuaki M, et al: Effects of acute coronary syndrome and stable coronary artery disease on bleeding and ischemic risk after percutaneous coronary intervention. Circ J. 2021;85: 1928-1941.
- Yamaji K, et al: Long-term outcomes after coronary stent implantation in patients presenting with versus without acute myocardial infarction (an observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Am J Cardiol 2015; 116: 15-23.
- Valgimigli M, et al: 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39: 213-260.
- Nakamura M, et al: JCS 2020 guideline focused update on anti-thrombotic therapy in patients with coronary artery disease. Circ J 2020; 84: 831-865.
- Valgimigli M, et al: Radial versus femoral access in patients with acute cor- nary syndromes undergoing invasive management: A randomized multicentre trial. Lancet 2015; 385: 2465-2476.
- Urban P, et al: Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 2019; 140: 240-261.
- Valgimigli M, et al: Effects of cobalt-chromium everolimus eluting stents or bare metal stent on fatal and non-fatal cardiovascular events: Patient level meta-analysis. BMJ 2014; 349: g6427.
CARDIOVASC 2023; 22(4): 46-50 (published on 28.11.23, ahead of print)