The antisense oligonucleotide nusinersen was approved in 2017 as the first drug for the causal treatment of patients with 5q-associated spinal muscular atrophy (5q-SMA) [1]. The drug targets the cause of the disease, SMN protein deficiency [1]. Extensive clinical and real-world studies have demonstrated that nusinersen can lead to stabilization and even improvement in motor function – in all age groups and SMA types I-III [2-7].
5q-SMA is an autosomal recessive progressive neomuscular disorder [8]. If left untreated, patients are at risk of progressive motor neuron degeneration, resulting in increasing muscle weakness and atrophy [8]. Until the introduction of causally effective drug therapy, the infantile form of 5q-SMA, in which symptoms become apparent in the first 6 months of life, was considered one of the most common genetic causes of death in infants and young children [8]. With nusinersen (SPINRAZA®), a therapy option is available that makes it possible to maintain or even increase muscle strength [1-7].
Nusinersen leads to the formation of higher amounts of SMN protein [9].
The cause of progressive denervation of skeletal muscle is a deficiency of the protein “survival motor neuron” (SMN), which is vital for motor neurons. Due to mutations or deletions in the SMN1 gene, too little SMN protein is produced in patients with 5q-SMA [8].
Nusinersen contributes to the production of more functional SMN protein, ensuring that motor neurons can survive [9]. This can slow or even halt the progression of the disease. Patients benefit from preservation or improvement of muscle function. The efficacy of nusinersen has been demonstrated for all age groups and disease severities: infants with presymptomatic 5q SMA, symptomatic children and adolescents, and adults [2-7].
Children with 5q-SMA benefit from presymptomatic therapy
In clinical trials, many children with 5q SMA treated with nusinersen achieved important motor milestones not observed in untreated patients with 5q SMA [2-4]. This showed that early SPINRAZA therapy can enable age-appropriate development in children with 5q-SMA. Most patients with genetically diagnosed 5q-SMA who were pre-symptomatically treated with nusinersen in the NURTURE clinical trial had reached motor milestones appropriate to their age at the interim evaluation of the study (Table 1) [10].
Table 1: Interim analysis of the NURTURE study in February 2020; mean age of study participants 3.8 years (n = 25). [10]
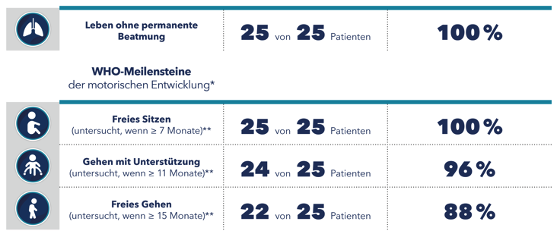





* Age at which healthy children normally reach these milestones.
** The time of the examination was within the time window specified by WHO. [11]
Significant improvements were also seen in bulbar functions, which are required for chewing and swallowing [12]. While many untreated patients with 5q-SMA tire when chewing, choke on food, or require a feeding tube [13-16], presymptomatic treatment with nusinersen in the NURTURE study resulted in 92% of children continuing to swallow and 84% managing without a feeding tube [12]. Accordingly, parents of children with 5q-SMA treated with nusinersen were generally not concerned about their children’s swallowing, feeding, and weight [12].
Efficacy of nusinersen also demonstrated in adults
Extensive data from observational studies of adult patients with 5q-SMA show that treatment with nusinersen can result in significant improvements in all motor function scales used (HFMSE, RULM, 6-minute walk distance) [5-7].
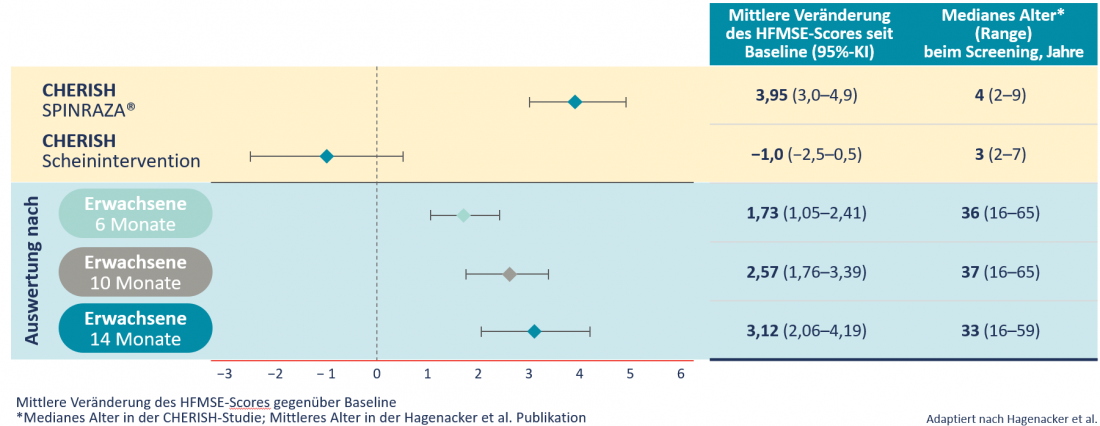
In a prospective, multicenter study involving 139 patients from 10 clinical centers in Germany, a high proportion of patients with 5q-SMA type II or III showed clinically relevant improvements in motor function. At all study time points, patients achieved statistically significant improvements in HFMSE score on average. This had increased by 3.12 points after 14 months. In the natural course of the disease, however, the HFMSE score decreases by an average of 0.5-1.0 points per year [6]. A comparable improvement of the HFMSE score was observed in adults as in children (Table 2) [6].
Table 2: Mean changes in HFMSE score since baseline: Comparison of 126 children with late 5q-SMA disease onset from the CHERISH study and 173 adult patients from the Hagenacker et al. Publication [3,6].

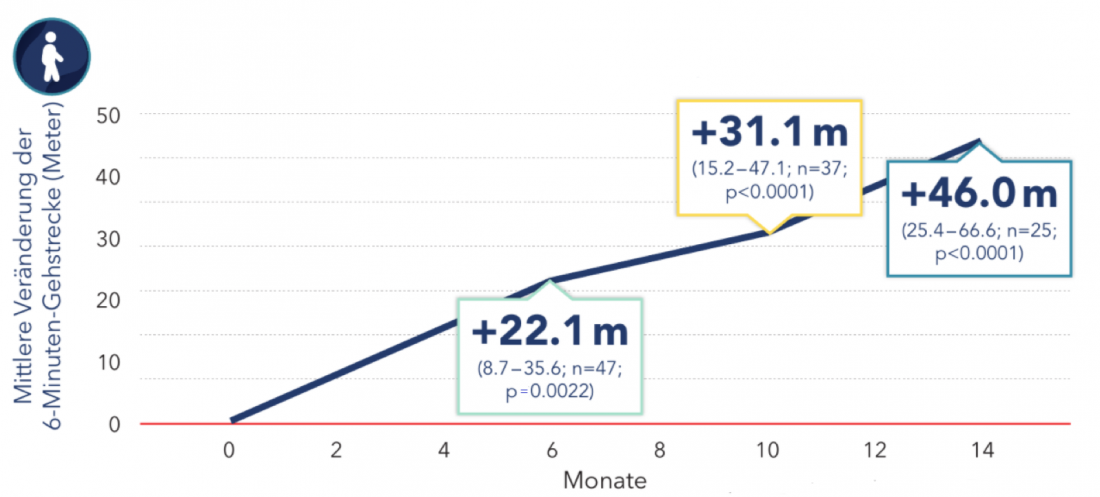
In the RULM score, which measures upper extremity function, the study showed a significant improvement of 1.09 points on average after 14 months. In addition, the patients managed to cover a mean of 403 meters within 6 minutes. They were thus able to walk an average of 46 meters further than before the start of therapy (Fig. 2) [6].
The age of the patients did not affect the results. However, the extent of the improvements depended on the patient’s residual motor function at baseline. The higher the level of residual motor function before the start of therapy, the higher the expected effect of nusinersen therapy [6].
Figure 2: Change in 6-minute walking distance in adults with nusinersen therapy. Boxes show mean and 95% CI [6]. Modified after Hagenacker T et al. [6].
Patients want to participate in normal life
Many adult patients desire to maintain their strength to sit, stand, and walk independently [17]. The functions of the hands and arms are indispensable for everyday tasks, such as being able to wash or dress oneself independently. Fine motor skills also play an important role in the social environment of patients, which manifests itself in the use of a smartphone or laptop.
However, in the natural course of 5q-SMA, there is progressive muscle weakness and loss of motor function [8]. Treatment with nusinersen may give adults with 5q SMA hope for the future. Not only can patients achieve longer-term stabilization of the functional status quo, many of them experience relevant improvements in motor function, have more strength in their hands and fingers, and are better able to stand up, stand longer, and walk farther [5-7].
Targeted effect – Central to the CNS
In doing so, Nusinersen targets the cause of 5q-SMA. It reversibly binds to the pre-mRNA of the paralogous SMN2 gene, which is targeted for SMN protein formation in patients with 5q-SMA. As a result, splicing factors are repressed and exon 7 remains in the mature SMN2 mRNA, allowing more functional SMN protein to be formed (Fig. 3) [1, 9].
Figure 3: Targeted mechanism of action of nusinersen [9].

Nusinersen is administered intrathecally via a lumbar puncture and thus goes directly to where it is supposed to work – the central nervous system [1]. Intrathecal application ensures that nusinersen is delivered directly to the central site of action where it can exert its targeted and specific effect on the pre-mRNA of the SMN2 gene [1]. The effects of nusinersen begin rapidly, as early as 1 to 3 days after injection [18]. The high specificity of nusinersen and its targeted application to the central nervous system have been shown to be safe in long-term use and also resulted in a sustained effect on motor function in a wide range of patients with 5q SMA [5-7,10,19-23].
At the same time, intrathecal use has advantages in terms of adherence. Data to date indicate that most patients are receiving their nusinersen doses as planned [24]. For other diseases, compliance has been shown to be higher for therapies administered in a clinic setting than for oral, daily therapies [25,26]. It is therefore reasonable to assume that because of intrathecal administration, physicians can be assured that patients are receiving the correct dose of nusinersen at the correct time and that their health status and response to treatment can be routinely monitored.
No systemic side effects expected
An important advantage of intrathecal use of nusinersen is compartment-specific exposure, which makes systemic side effects unlikely. This was confirmed in both clinical and real-world studies. Headache, back pain, and vomiting are the most commonly observed adverse events associated with intrathecal application [1]. They may occur shortly after nusinersen administration and are largely due to postpuncture syndrome, which can occur after lumbar punctures even without application of drug substances [6]. The safety profile of nusinersen is well documented over 7 years [19].
Application also possible for scoliosis
Even in cases of pronounced scoliosis or other severe spinal deformities, which are common in 5q SMA patients, intrathecal injection of nusinersen can generally be successful, safe, and rapid [27]. If necessary, injection into the CSF space should be performed under imaging guidance using computed tomography (CT) [27].
Conclusion
The central application of nusinersen provides a targeted effect immediately where it is needed. The efficacy of nusinersen has been demonstrated in extensive clinical trials and in real-world studies for all ages and severities of 5q SMA [2-7]. Adult patients with 5q-SMA may also benefit from therapy and achieve clinically significant stabilization or improvements in motor function [5-7].
The benefit of nusinersen is also reflected in patient satisfaction: in a prospective observational study of adult patients conducted in Germany, 95.7% reported satisfaction with nusinersen – regardless of age and disease severity [28].
In the meantime, there is extensive experience from everyday clinical practice. More than 11,000 patients worldwide have been treated with nusinersen to date [29].
Literature
- SPINRAZA® Technical Information, as of August 2019 (www.swissmedicinfo.ch)
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersenversus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N Eangl J Med. 2017;377:1723-32.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med. 2018;378:625-35.
- De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019;29:842-56.
- Walter MC, Wenniger S, Thiele S, et al. Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 – A Prospective Observational Study. J Neuromuscul Dis. 2019;6:453-65.
- Hagenacker T, Wurster CD, Günther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19:317-25.
- Maggi L, Bello L, Bonanno S, et al. Nusinersensafety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. 2020;91(11):1166-74.
- Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371:2120-33.
- Goodkey K, Ashley T, Maruyama R, et al. Nusinersen in the Treatment of Spinal Muscular Atrophy. Methods Mol Biol. 2018; 1828: 69-76.
- Swoboda KJ, Kirschner J, Finkel RS, et al; NURTURE Study Group. Nusinersen effect in infants who initiate treatment in a presymptomatic stage of SMA: NURTURE results. Cure SMA 2020 Virtual SMA Conference; June 8-12, 2020; https://curesma2020.biogenscicomm.com/curesma2020/sma/swoboda/#home. Retrieved September 2021.
- WHO Multicentre Growth Reference Study Group. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006; 450: 86 – 95.
- Swoboda KJ, Sansone VA, De Vivo DC, et al. Preserved swallowing function in infants who initiated nusinersen treatment in the presymptomatic stage of SMA: results from the NURTURE study. Presented at MDA Clinical and Scientific Conference 2021, March 15-18, 2021.
- Van der Heul AMB, Wijngaarde CA, Wadman RI, et al. Bulbar Problems Self-Reported by Children and Adults with Spinal Muscular Atrophy. J Neuromuscul Dis. 2019;6:361-8.
- Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810-7.
- Van der Heul AMB, Cuppen I, Wadman RI, et al. Feeding and Swallowing Problems in Infants with Spinal Muscular Atrophy Type 1: an Observational Study. J Neuromuscul Dis. 2020;7:323-30.
- Choi YA, Suh DI, Chae JH, et al. Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int J Pediatr Otorhinolaryngol. 2020;130:109818.
- Burbridge C, Kelly K, Garcia L, et al. PND74 Mapping a qualitative exploration of meaningful change in later-onset (type II or III) spinal muscular atrophy to the Hammersmith Functional Motor Scale Expanded (HFMSE). Value in Health. 2019; 22, Suppl 2, p284.
- Mazur C, Powers B, Zasadny K, et al. Brain pharmacology of intrathecal antisense oligonucleotides revealed through multimodal imaging. JCI Insight. 2019;4:e129240.
- Claborn MK, Stevens DL, Walker CK, et al. Nusinersen: A treatment for spinal muscular atrophy. Ann Pharmacother. 2019; 53(1): 61-69.
- Castro D, Finkel RS, Farrar MA, et al. Nusinersen in infantile-onset spinal muscular atrophy: results from longer-term treatment from the open-label SHINE extension study. Neurology. 2020; 94(15):1640.
- Chiriboga CA, et al. Longer-term treatment with nusinersen: results in later-onset spinal muscular atrophy from the shine study. Presented at AAN 2020; 25. April-1. May 2020; https://www.neurologylive.com/view/nusinersen-shows-efficacy-in-infantile-and-lateonset-spinal-muscular-atrophy; Retrieved September 2021.
- Acsadi G, Crawford TO, Müller-Felber W, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle Nerve. 2021;63:668-77.
- Duong T, Wolford C, McDermott, MP, et al. Nusinersen Treatment in Adults With Spinal Muscular Atrophy. Neurol Clin Pract. 2021; 11(3): e317-27.
- Paradis A, et al. Nusinersen experience in later onset spinal muscular atrophy. Presented at WMS 2020.
- Seal BS, Anderson S, Shermock KM, et al. Factors Associated with Adherence Rates for Oral and Intravenous Anticancer Therapy in Commercially Insured Patients with Metastatic Colon Cancer. J Manag Care Spec Pharm. 2016;22:227-35.
- Moran K, Null K, Huang Z, et al. Retrospective Claims Analysis Indirectly Comparing Medication Adherence and Persistence Between Intravenous Biologics and Oral Small-Molecule Therapies in Inflammatory Bowel Diseases. Adv Ther. 2019;36:2260-72.
- Cordts I, Lingor B, Friedrich P, et al. Intrathecal nusinersen administration in adult spinal muscular atrophy patients with complex spinal anatomy. Ther Adv Neurol Disord 2020; 13: 1756286419887616.
- Meyer T, Maier, A, Uzelac Z, et al. Treatment expectations and perception of therapy in adult patients with spinal muscular atrophy receiving nusinersen. Eur J Neurol. 2021; 28(8): 2582-95.
- New Data at Cure SMA 2021; Biogen, https://investors.biogen.com/news-releases/news-release-details/new-data-cure-sma-2021-highlight-long-term-efficacy-spinrazar; Last Access: September 2021.
Abbreviated SPINRAZA® Z: One vial contains 12 mg nusinersen in 5 ml artificial cerebrospinal fluid. I: Treatment of 5q-associated spinal muscular atrophy (SMA) D: Intrathecal application by lumbar puncture (LP). 4 Saturation doses of 12 mg (5ml) per application on days 0, 14, 28, 63; maintenance therapy every 4 months 12 mg (5ml). Spinraza may only be administered in hospital-based, specialized neuromuscular centers. It is mandatory that the treating medical personnel have experience in diagnosing and treating patients with spinal muscular atrophy and in performing LP. KI: Hypersensitivity to active substance or excipients. VM: No long-term safety data. Risk of side effects associated with LP. Difficulties of LP in very young patients/patients with scoliosis. Examination of platelets and coagulation before and regularly during therapy. Monitor urine protein before and regularly during therapy. ECG before start and regularly during therapy. Possible neurotoxicity at high doses and/or long-term use. Consider investigation for hydrocephalus in patients with impaired consciousness. S: Not recommended during S. UW: Infantile form: Respiratory infections, nasopharyngitis, urinary tract infections, ear infections, influenza, constipation, flatulence, weight loss, rash. Later onset of illness: fever, headache, vomiting, epistaxis, respiratory congestion, seasonal allergy, back pain, fall. Possible reduction in growth. Anti-drug antibody (ADA) incidence low, no apparent impact of ADA development on clinical response, adverse events, or pharmacokinetic profile of nusinersen noted. Postmarketing: serious infections such as meningitis, communicating hydrocephalus, aseptic meningitis, hypersensitivity (e.g., angioedema, urticaria, rash). IA: No interaction with CYP450, interaction due to competitive plasma protein binding, competitive effect or inhibition of transporters low. List A. The complete technical information is published at www.swissmedicinfo.ch. Biogen Switzerland AG, 6340 Baar. Status of the information: August 2019. Biogen-29360_09.2020
Biogen Switzerland AG
Neuhofstrasse 30
6340 Baar
www.biogen.ch
Biogen-128051_09.2021