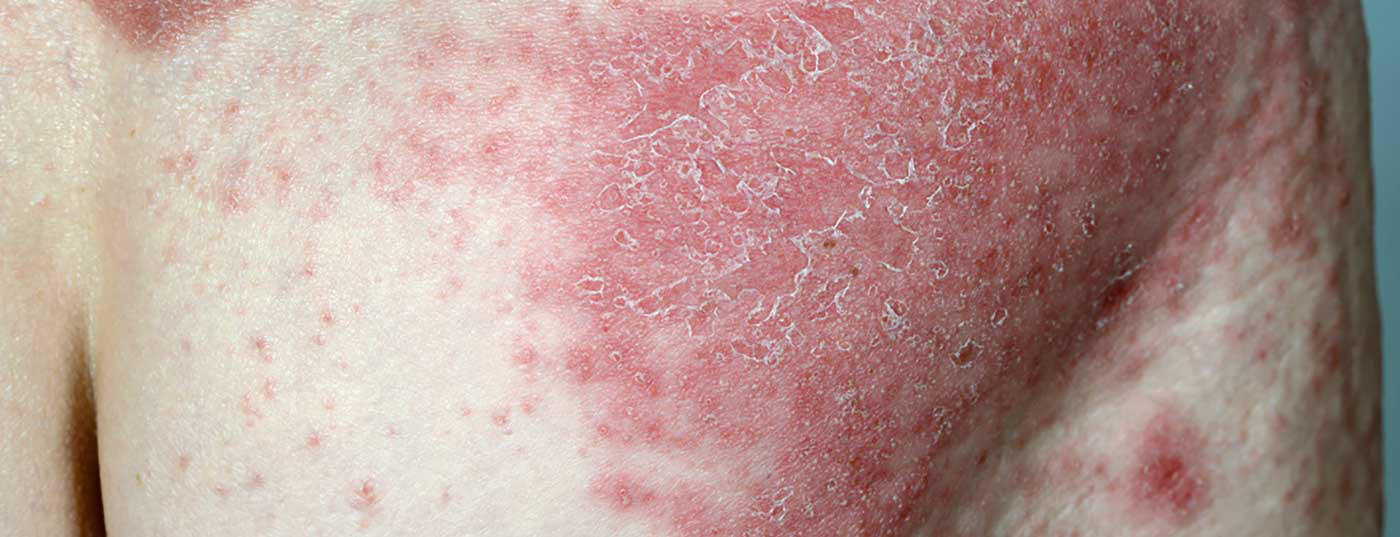
As in today’s era of personalized medicine a treatment tailored to the individual needs of psoriasis patients is important, there are several arguments for a use of small molecule substances. A look at current clinical data from studies in patients with moderate to severe plaque psoriasis is worthwhile.
An important treatment goal is to minimize symptoms and improve disease-related quality of life. Unlike biologics, whose targets are cytokines, the therapeutic target of Small Molecules is intracellular and the dosage form is oral or topical. Another difference is that biologics act very specifically, which is somewhat less the case with small molecules. In addition to the treatment options apremilast (Otezla®) or tofacitinib (Xelianz®), which have been approved for some time, further small molecule agents are currently being researched. These include the topical PDE4 inhibitor roflumilast, as well as the TYK-2 inhibitor deucravacitinib and the RIPK-1 inhibitor GSK2982772 (Tab. 1). On the one hand, topical formulations offer an anti-inflammatory alternative to cortisone-containing topical preparations, and on the other hand, a therapy trial with small molecules can be helpful for patients who do not respond adequately to biologics or who prefer oral medication, according to Prof. Dr. med. Enikö Sonkoly from the Karolinska Institute, Stockholm (S) [1]. It is also an interesting treatment option for special localizations such as palmoplantar areas or scalp, as well as for joint involvement.
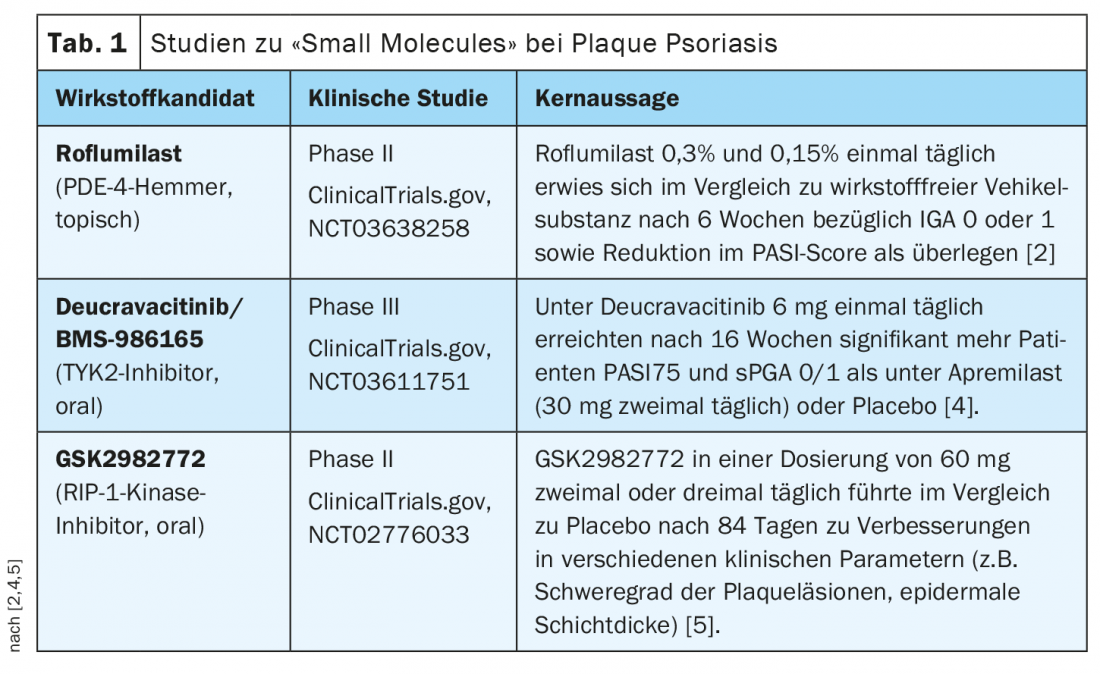
Overview of current study data
Roflumilast: In the three-arm randomized double-blind phase II study in 331 adult patients with plaque psoriasis, a once-daily topical formulation of roflumilast 0.3% and roflumilast 0.15% was compared with an active-agent-free vehicle [2,3]. Six weeks after baseline, 28% and 23% of study participants in the two roflumilast groups, respectively, achieved appearance-free or near-appearance-free skin (IGA 0 or 1), compared with 8% in the placebo group. The PDE-4 inhibitor was also superior in terms of PASI response in the placebo comparison; after six weeks of treatment, there was a reduction in PASI score of approximately 50% in both potencies compared to 18% with placebo. With regard to local side effects, there was no difference in the three study arms.
Deucravacitinib (BMS-986165): The orally administered, selective tyrosine kinase 2 (TYK2) inhibitor was evaluated in two multicenter, randomized, double-blind phase III trials. POETYK PSO-1 and POETYK PSO-2 evaluated safety and efficacy of deucravacitinib (6 mg once daily) compared with placebo and apremilast (30 mg twice daily) in patients with moderate-to-severe plaque psoriasis [4]. The POETYK PSO-1 study enrolled 666 patients. After a 16-week treatment period, more patients achieved a PASI75 and static Physician’s Global Assessment (sPGA) score of 0 or 1 with deucravacitinib than with apremilast and placebo. The POETYK PSO-2 study evaluated efficacy and safety of deucravacitinib in a total of 1020 patients. After 16 weeks of treatment, deucravacitinib also proved superior to apremilast or placebo in terms of PASI75 and sPGA 0/1 response rates. The overall safety profile of deucravacitinib proved consistent with previously reported results.
GSK2982772: Data are available on this RIP-1 kinase inhibitor from a multicenter, randomized, double-blind, placebo-controlled phase II trial [5]. 65 psoriasis patients were randomized to the following three study arms: GSK2982772 at a dose of 60 mg twice daily or three times daily, or placebo. The treatment period was 84 days. GSK2982772 was shown to be safe and well tolerated, and clinical improvements were noted indicating that RIP-1 kinase inhibition can favorably influence inflammatory processes in plaque psoriasis. Treatment with GSK2982772 resulted in a reduction in epidermal layer thickness and infiltration by CD3+ T cells compared with placebo, and plaque lesion severity (“plaque lesion severity sum”) improved compared with placebo. Interpretation of the results of the three-times-daily application proved to be complex, as the placebo response was high. Overall, the results support further studies in this indication area.
Literature:
- Sonkoly E: Existing and upcoming small molecules. Inflammatory diseases, Prof. Enikö Sonkoly, MD, Psoriasis, D1T02.2, EADV Annual Meeting, Oct. 29, 2020.
- Lebwohl MG, et al: Trial of Roflumilast Cream for Chronic Plaque Psoriasis. N Engl J Med 2020; 383: 229-239.
- Siebenand S: Psoriasis study. Pharmazeutische Zeitung (Online), 23.07.2020, www.pharmazeutische-zeitung.de (last accessed 17.02.2021).
- “Positive topline results from second pivotal phase 3 study show superiority of deucravacitinib in plaque psoriasis compared with placebo and Otezla (apremilast),” https://arznei-news.de/deucravacitinib-plaque-psoriasis/#a1
- Weisel K, et al: Clinical Pharmacol Response to Inhibition of Receptor-Interacting Protein Kinase 1 (RIPK1) in Active Plaque Psoriasis: A Randomized Placebo-Controlled Study. Pharmacology & Therapeutics 2020; 108(4): 808816.
DERMATOLOGY PRACTICE 2021; 31(2): 48